
�۲�Ƚ��������ѧϰ��ѧ����Ҫ��������������������ѧ����ʽ��
2Mg+O2  2MgO��2H2+O2
2MgO��2H2+O2  2H2O��2CO+O2
2H2O��2CO+O2 2CO2��
2CO2��
��1��ͨ���Ƚϣ��������������ͬ�㣬����д���������㣺
�� ;
�� .
(2) ���ϻ�ѧ����ʽҲ�����֮ͬ��������д������һ������������һ�㲻֮ͬ
���� ��
��3������ѧϰCu2(OH)2CO3 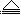 2CuO+H2O+CO2������ᷢ����������������
2CuO+H2O+CO2������ᷢ����������������
Ӧ���ƣ�������֮���� ��
 ��ְٷְټ���ϵ�д�
��ְٷְټ���ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��������Ϊ������������ȼ�ϣ�����㷺���õĻ�ʯȼ�������ʲô�ŵ㣿�������㼴�ɣ�
a����_________����b����_________����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����죬С��������ҵ����������һ�����緹��������������緹�������⣬�������ҳ��������Ǵ��ŹǺ��㻨����
��1��Ϊ�˾���Ӫ��������Ϊ��Ӧ�����ӵ�һ��������_________����
��2��С�������轫��ϴ�á��кú������ˣ���ϴ��ʱ�������ҵ������������ˣ�Ϊ�˷�ֹ�������⣬������Ľ�������_________����
��3����ϴ�ˡ�����������ʱ���õ���ˮ������ʹ�õ�ˮ�Ǿ�������ˮ���������ģ�ˮ�ľ�������������������̿�ȣ���Ŀǰ�㷺��������ˮ�������Ǽ����������������ۡ�ɱ���ȹ�����һ����������������ƣ�Na2FeO4����������������Ԫ�صĻ��ϼ�Ϊ��_________����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���ж������غ㶨�ɵ�����������( )
A. �����غ㶨��ֻ�����ڻ�ѧ��Ӧ���������������仯
B�������غ㶨���о������ݽ���ָ���� �����������ƹ㵽����������
�����������ƹ㵽����������
C���������Ըı��������ʵĻ�ѧ��Ӧ���ʣ��� �����μӵķ�ӦҲ���������غ�
�����μӵķ�ӦҲ���������غ�
����
D������ȼ�պ��������ᣬ�����������غ㶨��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��20g H2��O2��N2�Ļ�������ȼ����ȫ��Ӧ������18gˮ����ʣ�����岻������( )
A��N2��H2�� ��B��O2��N2 C��3g N2���� ��D��H2��O2��N2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����ͨ��״���£�������û����ɫ��û����ζ�����塱����仰��ָ�����ģ�������
A. �������� B. �����仯 C. ��ѧ���� D. ��ѧ�仯
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ijͬѧ�������ȼ�ս���̽����
�ٵ�ȼһ֧������һֻ�ձ�������ȼ�ŵ������ϣ�����ȼ��Ƭ�̺�Ϩ�� ����ʵ��һ��
����ʵ��һ��
���ٵ�ȼ����Ȼ���𣬿�����о����һ�ư��̣��û���ȼ���̣��������±���ȼ����ʵ�����
�۽���ϸ���ȵ�ľ��ˮƽ��������Ļ����ϣ��Լ��Ⱥ�۲�ľ��ȼ��������ʵ������

��1���������У�������ȼ��Ƭ�̵�ԭ����_________________________________________��
��2���������У����ڰ��̵ijɷ֣���ͬѧ���������в��룺A.���� ��ˮ������B.������ʯ������С������C.�����Ƕ�����̼������Ϊ���������е������ǣ�����ţ�____________��������________________________________________��
��ˮ������B.������ʯ������С������C.�����Ƕ�����̼������Ϊ���������е������ǣ�����ţ�____________��������________________________________________��
��3����ͬѧ����ʵ��һ�Ĺ����У��������ڻ����Ϸ����ձ��ڱڱ�Ѭ�ڣ�����Ϊ�������������в����ʵ��ǣ� ��
A������ʵ�飬���۲��Ƿ�����ͬ����
B���������ϣ��˽�ʯ������Ҫ�ɷ֣�̽�����ɵĺ�ɫ������ʲô
C����Ϊ�뱾��ʵ��Ŀ���أ���������
D��ѯ����ʦ��ͬѧ���������ɺ�ɫ���ʵ�ԭ��
��4��ľ����������IJ������ȱ�ڣ�˵������������¶���______________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���ݴ��������������ʵ�ȥ������Һ������ʵ��ͼʾ�ش�������⣺
��1���������������������������
��2�������բڢۢٲ�������50g16%��NaCl��Һ����������Һ���������������������ƫ����ƫС������Ӱ�족����
��3����������ֹͣ������ʱ��������������ţ���
A����������Һ����ȫ����ʱ B�����������д�����������ʱ
��4����ȥ�����������Ե����ʲ����㾫�εIJ��ʣ�����ȷ��������Ϊ�ڢۢ٢ܢݢڣ����²����п��ܻᵼ�¾��β��ʣ�����= ��100%��ƫС��������������ţ���
��100%��ƫС��������������ţ���
A������������������Һ�����ձ� B�������������Ӷ���
C����������Һ�������ֽ��Ե D����������û��ʹ�ò�������裮
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com