
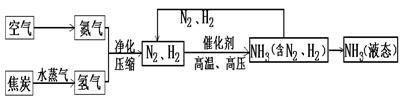
ŗĻ³É°±ŹĒČĖĄąæĘѧ¼¼ŹõÉĻµĆŅ»ĻīÖŲ“óĶ»ĘĘ£¬¶ŌÉē»į·¢Õ¹Óė½ų²½×ö³öĮĖ¾Ž“ó¹±Ļ×”£ŗĻ³É°±µÄ¹¤ŅÕĮ÷³ĢĶ¼ČēĻĀ£ŗ
£Ø1£©Į÷³ĢÖŠÉś³ÉµÄ°±Ęų£¬ŌŚÅ©ŅµÉĻŹĒŗĻ³É ·Ź£ØĢī»Æ·ŹµÄÖÖĄą£©µÄŌĮĻ”£
£Ø2£©ÖĘČ”ĒāĘųµÄ·“Ó¦¹ż³ĢĪŖ£ŗ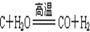 £¬
£¬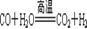 ”£
ӣ
ÉĻŹöĮ½øö»Æѧ·½³ĢŹ½ÖŠ±ķĻÖŃõ»ÆŠŌµÄĪļÖŹŹĒ £ØĢī»ÆѧŹ½£©”£
£Ø3£©ŌĮĻÖŠN2”¢H2ÖŠ³£ŗ¬ÓŠCO2ŗĶĖ®ÕōĘų£¬·“Ó¦Ē°ŅŖ¾»»Æ”£¾»»Æ¹ż³ĢÖŠĖłÓĆĮ½ÖÖŹŌ¼ĮµÄĻČŗóĖ³ŠņĪŖ ”£
£Ø4£©Š“³öN2”¢H2·“Ӧɜ³ÉNH3µÄ»Æѧ·½³ĢŹ½ ”£
£Ø5£©øĆĮ÷³ĢÖŠ²śÉśæÉŃ»·Ź¹ÓƵÄĪļÖŹŹĒ ”£
¢ÅµŖ·Ź ¢ĘH2O ¢ĒĻČ°ŃŌĮĻĘųĶعżNaOHČÜŅŗ£Ø»ņŹÆ»ŅĖ®µČ¼īŅŗ£©£¬ŌŁĶعżÅØĮņĖį£Ø»ņ¼īŹÆ»ŅµČøÉŌļ¼Į£© ¢Č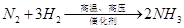 ¢ÉN2 H2
¢ÉN2 H2
”¾½āĪö”æ
ŹŌĢā·ÖĪö£ŗ¢Å°±ĘųÖŠŗ¬ÓŠµŖŌŖĖŲ£¬æÉŅŌ×öµŖ·Ź£»£Ø2£©øų³öŃõŌŖĖŲµÄŹĒŃõ»Æ¼Į£¬ĖłŅŌŃõ»Æ¼ĮĪŖĖ®£»£Ø3£©ĻČĶØČėĒāŃõ»ÆÄĘČÜŅŗ³żČ„¶žŃõ»ÆĢ¼ŌŁĶØČėÅØĮņĖįÖŠ³żČ„Ė®ÕōĘų£»£Ø4£©×¢ŅāĢõ¼ž£¬øßĪĀ”¢øßŃ¹”¢“߻ƼĮ£»£Ø5£©ÓÉĮ÷³ĢĶ¼æÉÖŖ£¬øĆĮ÷³ĢÖŠ²śÉśæÉŃ»·Ź¹ÓƵÄĪļÖŹŹĒN2 H2”£
æ¼µć£ŗŗĻ³É°±
µćĘĄ£ŗ“ĖĢāæ¼²ģÖŖŹ¶µć½ĻĪŖÄ°Éś£¬µ«×ŠĻøÉóĢāæÉÖŖæ¼²ģµÄĖ¼ĻėŗĶ»ł±¾ÖŖŹ¶µć¶¼ŹĒÖŠæ¼ŅŖĒóµÄ»ł±¾ÖŖŹ¶£¬“ĖĢāÓŠŅ»¶ØµÄÄŃ¶Č£¬ŅŖ¶ą¶ĮĢį£¬¶ąĮŖĻµĢāøÉ”£


 ĆūŠ£æĪĢĆĻµĮŠ“š°ø
ĆūŠ£æĪĢĆĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ

| ||
| ||
| ||
| øßĪĀøßŃ¹ |
| ||
| øßĪĀøßŃ¹ |
| ĪļÖŹ | H2 | N2 | O2 | NH3 |
| ·Šµć | -252”ę | -195.8”ę | -183”ę | -33.35”ę |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ

| ||
| ||
| øßĪĀøßŃ¹ |
| ||
| øßĪĀøßŃ¹ |
| ĪļÖŹ | H2 | N2 | O2 | NH3 |
| ·Šµć | -252”ę | -195.8”ę | -183”ę | -33.35”ę |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā

 CO+H2£¬CO+H2O
CO+H2£¬CO+H2O CO2+H2£®ÉĻŹöĮ½øö»Æѧ·½³ĢŹ½ÖŠ±ķĻÖŃõ»ÆŠŌµÄĪļÖŹŹĒ________£ØĢī»ÆѧŹ½£©£®
CO2+H2£®ÉĻŹöĮ½øö»Æѧ·½³ĢŹ½ÖŠ±ķĻÖŃõ»ÆŠŌµÄĪļÖŹŹĒ________£ØĢī»ÆѧŹ½£©£®| ĪļÖŹ | H2 | N2 | O2 | NH3 |
| ·Šµć | -252”ę | -195.8”ę | -183”ę | -33.35”ę |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ŗĻ³É°±ŹĒČĖĄąæĘѧ¼¼ŹõÉĻµĆŅ»ĻīÖŲ“óĶ»ĘĘ£¬¶ŌÉē»į·¢Õ¹Óė½ų²½×ö³öĮĖ¾Ž“ó¹±Ļ×”£ŗĻ³É°±µÄ¹¤ŅÕĮ÷³ĢĶ¼ČēĻĀ£ŗ

£Ø1£©ŗĻ³É°±ŠčŅŖµÄµŖĘųĄ“×ŌæÕĘų£¬æÕĘųÖŠµŖĘųµÄĢå»ż·ÖŹżŌ¼ĪŖ”””””” ”£
”” £Ø2£©ÖĘČ”ĒāĘųµÄ·“Ó¦¹ż³ĢĪŖ£ŗC+H2O![]() CO+H2£¬CO+H2O
CO+H2£¬CO+H2O![]() CO2+H2 ”£ÉĻŹöĮ½øö»Æѧ·½³ĢŹ½ÖŠ±ķĻÖŃõ»ÆŠŌµÄĪļÖŹŹĒ”””””””” £ØĢī»ÆѧŹ½£©”£
CO2+H2 ”£ÉĻŹöĮ½øö»Æѧ·½³ĢŹ½ÖŠ±ķĻÖŃõ»ÆŠŌµÄĪļÖŹŹĒ”””””””” £ØĢī»ÆѧŹ½£©”£
”” £Ø3£©ŌĮĻÖŠN2”¢H2ÖŠ³£ŗ¬ÓŠCO2ŗĶĖ®ÕōĘų£¬·“Ó¦Ē°ŅŖ¾»»Æ”£¾»»ÆµÄ¹ż³ĢŹĒ””””””””
”””””””””””””””””””””””””””””””””””””” ”£
”” £Ø4£©Š“³öN2”¢H2·“Ӧɜ³ÉNH3µÄ»Æѧ·½³ĢŹ½”””””””””””””””””””””””””” ”£
”” £Ø5£©½«·Šµć²»Ķ¬µÄĘųĢå·ÖĄėæŖĄ“£¬³£²ÉÓĆŅŗ»Æ·ÖĄė·Ø”£Čē£¬æŲÖĘĪĀ¶ČŌŚ-183”ꏱ£¬æɽ«æÕĘųÖŠN2ÓėO2·ÖĄė”£øł¾ŻĻĀ±ķÖŠĪļÖŹµÄ·ŠµćÅŠ¶Ļ£¬ŅŖ½«²śĪļNH3ÓėN2”¢H2·ÖĄėæŖĄ“£¬×īŹŹŅĖµÄĪĀ¶ČÓ¦øĆæŲÖĘŌŚ”””””””””” ”ę”£
ĪļÖŹ | H2 | N2 | O2 | NH3 |
·Šµć | -252”ę | -195.8”ę | -183”ę | -33.35”ę |
””””
””
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ2012Äź¹ć¶«Ź”·šÉ½ŹŠÖŠæ¼»ÆѧŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗ½ā“šĢā

 CO+H2£¬CO+H2O
CO+H2£¬CO+H2O CO2+H2£®ÉĻŹöĮ½øö»Æѧ·½³ĢŹ½ÖŠ±ķĻÖŃõ»ÆŠŌµÄĪļÖŹŹĒ______£ØĢī»ÆѧŹ½£©£®
CO2+H2£®ÉĻŹöĮ½øö»Æѧ·½³ĢŹ½ÖŠ±ķĻÖŃõ»ÆŠŌµÄĪļÖŹŹĒ______£ØĢī»ÆѧŹ½£©£®²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com