
| ��Ҫ�ɷ֣�mg/L���� ̼�������HCO3-����173-205 �����ӣ�Cl-����1.0-8.0 �������SO42-����16.08-19.52 �����ӣ�Na+����8-50 þ���ӣ�2.5-12.5 PHֵ��7.8��0.5��1��þ���ӻ�ѧ���ſɱ�ʾΪ Mg2+ ����SO42-�������֡�2���ĺ�����һ����������Ӵ�������λ�ĸ���� ����2���ÿ�Ȫˮ�� �� �ԣ���ᡱ��������С�������3���ճ��������� ����ˮ ������ˮ��Ӳˮ����ͨ����� ��������ˮ��Ӳ�ȣ�
��������1���������ӵı�ʾ�������ڱ�ʾ�����ӵ�Ԫ�ط������Ͻǣ���������������������������������ǰ�����������ں�1�����ʱ��1Ҫʡ�ԣ�����ʾ��������ӣ�������Ԫ�ط���ǰ������Ӧ�����֣����н�𣻸��ݱ���Ԫ�ط������Ͻǵ����ֱ�ʾ������������������н�� ��2������PH��7����Һ�Լ��ԣ����н�� ��3�������ճ�������������ˮ��Ӳˮ�� ����ˮ������ˮ��Ӳ�ȵķ�������У����н�� ����⣺��1���������ӵı�ʾ�������ڱ�ʾ�����ӵ�Ԫ�ط������Ͻǣ���������������������������������ǰ�����������ں�1�����ʱ��1Ҫʡ�ԣ�����ʾ��������ӣ�������Ԫ�ط���ǰ������Ӧ�����֣���þ���� ��ʾΪ��Mg2+�� ���ݱ���Ԫ�ط������Ͻǵ����ֱ�ʾ������������������SO42-�������֡�2���ĺ����ǣ�һ����������Ӵ�������λ�ĸ���ɣ� �ʴ�Ϊ��Mg2+��һ����������Ӵ�������λ�ĸ���ɣ� ��2������PH��7����Һ�Լ��ԣ��б�ǩ��֪PHֵ��7.8��0.5����� �ÿ�Ȫˮ�� ���ԣ��ʴ�Ϊ��� ��3�������ճ�������������ˮ��Ӳˮ�� ����ˮ������ˮ��Ӳ�ȵķ�������У� �ʴ�Ϊ������ˮ����У� ���������⿼��ѧ���Ի�ѧ�������ȷ��д�ͻ�ѧ֪ʶ���ճ������е�Ӧ�ã�  
��ϰ��ϵ�д�
 �����ҵ��ٿ���������������ϵ�д� �����ҵ��ٿ���������������ϵ�д�
���ϰ��
��Ŀ�����л�ѧ ��Դ�� ���ͣ� 25��ij��Ȼ��Ȫˮ����Ҫ�ɷ����£��������Ķ�����գ�  ��1����SO42-�������֡�2���ĺ����� ��2����λ����ɵ���������� ����2���ÿ�Ȫˮ�� �� �ԣ���ᡱ��������С�������3���ճ��������� ����ˮ ������ˮ��Ӳˮ����ͨ��
������� ��������ˮ��Ӳ�ȣ��鿴�𰸺ͽ���>> ��Ŀ�����л�ѧ ��Դ�� ���ͣ� 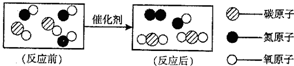 ��1����ͼ��ת������β�����к��������ʾ��ͼ�� ��1����ͼ��ת������β�����к��������ʾ��ͼ���ٷ�Ӧ���ͼʾ�к��� 3 3 �ַ��ӣ���ͼ����ʾ�Ļ�ѧ����ʽ 2CO+2NO
2CO+2NO ��
�۴�ͼ���㻹�ܻ�ȡ����Ϣ�� �ڻ�ѧ�仯�з��ӿ����ٷ֣���ѧ��Ӧǰ��Ԫ�ص�����䣬�������ԭ�ӹ��ɣ��������� �ڻ�ѧ�仯�з��ӿ����ٷ֣���ѧ��Ӧǰ��Ԫ�ص�����䣬�������ԭ�ӹ��ɣ��������� ������һ�֣���2��ij��Ȼ��Ȫˮ����Ҫ�ɷ����±����������Ķ�����գ�
���ճ������г���______������ˮ��Ӳˮ�� ��ũҵ���ֽ���ֲ��ʱ������ˮ�����Ϊ��ࡢ�ι��Ŀ����______�� �鿴�𰸺ͽ���>> ��Ŀ�����л�ѧ ��Դ��2012��㶫ʡ÷����ѧ�п���ѧģ���Ծ��������棩 ���ͣ������ ��1����ͼ��ת������β�����к��������ʾ��ͼ�� �ٷ�Ӧ���ͼʾ�к��� �ַ��ӣ� ��ͼ����ʾ�Ļ�ѧ����ʽ �� �۴�ͼ���㻹�ܻ�ȡ����Ϣ�� ������һ�֣� ��2��ij��Ȼ��Ȫˮ����Ҫ�ɷ����±����������Ķ�����գ�
���ճ������г��� ������ˮ��Ӳˮ�� ��ũҵ���ֽ���ֲ��ʱ������ˮ�����Ϊ��ࡢ�ι��Ŀ���� �� 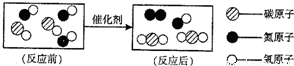 �鿴�𰸺ͽ���>> ͬ����ϰ��� ����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר�� Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com��Ȩ��������վ�������£�ͼƬ��Դ�����磬����Ȩ����Ȩ��ԭ�������У�ת�������ַ���Ȩ��������Ȩ����������������֪�����ǽ����촦������ϵqq��3310059649�� ICP�������: ��ICP��07509807��-10 ����������42018502000812�� | |||||||||||||||||||||||||||||||