
���� ��1���������������ɣ�
��2������ά����C�Ļ�ѧʽ���м��㼴�ɣ�
��3�����ݻ�ѧ����ʽ��ǡ����ȫ��Ӧʱ���ĵ���������г�����ʽ���Ϳɼ����20.00g��֭��ά����C��������Ȼ���������������ʽ���㼴�ɣ�
��� �⣺��1�������������֪��������VcΪ��ɫ��ɫ�ᾧ������ζ������ζ����Vc���������ʣ������ɫ��ɫ�ᾧ������ζ������ζ��
��2��ά����C�и�Ԫ�ص������ȣ�C��H��O=��12��6������1��8������16��6��=6��1��12�����6��1��12��
��3����20.00g��֭��ά����C������Ϊx
C6H8O6+I2=C6H6O6+2HI
176 254
x 25.40g��1.00%
$\frac{176}{254}=\frac{x}{25.40g��1.00%}$
x=0.176g
����C����������=$\frac{0.176g}{20.00g}$��100%=0.88%��
�𣺳�֭��ά����C����������Ϊ0.88%��
���� ������Ҫ����ѧ�����û�ѧʽ����ѧ����ʽ���м�������������ݻ�ѧ����ʽ������һ�����ʵ���������������������ʵ�������


 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����

| A�� | a���ʾt2��ʱ100g������Һ������ܽ������25g | |
| B�� | ��t2��ס��ҵı�����Һ���������ܼ�������������� | |
| C�� | t1��ʱ���ס��������ʵı�����Һ�к����ʵ�������� | |
| D�� | �����¶ȿɽ��ı�����Һ��ɲ�������Һ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �л���һ������̼Ԫ�أ����Ժ���̼Ԫ�صĻ�����һ�����л��� | |
| B�� | �û���Ӧ�е������ɣ����е������ɵķ�Ӧһ��Ϊ�û���Ӧ | |
| C�� | ����Һ�Լ��ԣ������Լ��Ե���Һһ���Ǽ���Һ | |
| D�� | �����������ƾ�����ˮ�ԣ����Թ����������ƿ�����ijЩ����ĸ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ������ˮ��������ԭ��CO2+H2O=H2CO3 | |
| B�� | ��ϡ�����ȥ���������⣺FeO+2HCI=FeCl2+2H2O | |
| C�� | ����������Ȼ����ȼ�ϣ�C2H5OH+3O2$\frac{\underline{\;��ȼ\;}}{\;}$2CO2+3H2O | |
| D�� | ��ҵ������ʯ�����ռCa��OH��2+2NaNO3=Ca��NO3��2+2NaOH |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 2O��ʾ���������� | B�� | �����ӵķ���ΪNa+ | ||
| C�� | �Ȼ����Ļ�ѧʽFeCl2 | D�� | H2O�к��������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
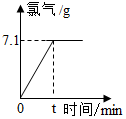 �Ȼ�����һ����Ҫ�Ļ���ԭ�ϣ�����Ȼ�����Һ���Ƶ��������������Ƶ����ʣ������Ļ�ѧ��Ӧ���£�2NaCl+2H2O $\frac{\underline{\;ͨ��\;}}{\;}$ Cl2��+H2��+2NaOH ��ȡһ��������������������Ϊ10%���Ȼ�����Һ���е�⣬���Ȼ�����ȫ��Ӧʱ��ֹͣͨ�磮���������������뷴Ӧʱ��Ĺ�ϵ��ͼ��ʾ������㣺
�Ȼ�����һ����Ҫ�Ļ���ԭ�ϣ�����Ȼ�����Һ���Ƶ��������������Ƶ����ʣ������Ļ�ѧ��Ӧ���£�2NaCl+2H2O $\frac{\underline{\;ͨ��\;}}{\;}$ Cl2��+H2��+2NaOH ��ȡһ��������������������Ϊ10%���Ȼ�����Һ���е�⣬���Ȼ�����ȫ��Ӧʱ��ֹͣͨ�磮���������������뷴Ӧʱ��Ĺ�ϵ��ͼ��ʾ������㣺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� |  ��ȼ�ƾ��� | B�� |  ������������ | ||
| C�� |  ��ȡҺ����� | D�� |  ����������ʹ��Ͳ�½� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ���� | B�� | ֬�� | C�� | ������ | D�� | ������ |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com