
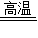 2Fe2O3+8SO2
2Fe2O3+8SO2


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012������ʡ��ɽ�о��꼶��һ���п�ģ�⿼�Ի�ѧ�Ծ����������� ���ͣ��ʴ���
��6�֣����������־��һ�����һ���ҵ��չ��ˮƽ����ҵ�����������Ҫ����Ϊ��
�����ջ�����4FeS2+11O2 2Fe2O3 + 8SO2
2Fe2O3 + 8SO2
��SO2ת��ΪSO3 ����ŨH2SO4����SO3 ��ش�������⣺
��1������������̢����γɵĹ���ʣ��� ��
��2��SO2������CO2���ƵĻ�ѧ���ʡ������������ʯ������β���е�SO2����д���˷�Ӧ�Ļ�ѧ����ʽ ��
��3���ڢڲ���Ӧ��һ�����Ϸ�Ӧ�����ж�ʵ�ִ˷�Ӧ����һ��Ӧ���� ��
��4����SO2��Cl2ͨ��ˮ����ǡ����ȫ��Ӧ���������ֳ������ᣬ��������л�ѧ����ʽ��Cl2+SO2+2H2O=== +2HCl
��5��ʵ����ϡ��Ũ����ķ����ǣ�
��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ������ʡ��ɽ�о��꼶��һ���п�ģ�⿼�Ի�ѧ�Ծ��������棩 ���ͣ������
��6�֣����������־��һ�����һ���ҵ��չ��ˮƽ����ҵ�����������Ҫ����Ϊ��
�����ջ�����4FeS2+11O2 2Fe2O3 + 8SO2
2Fe2O3 + 8SO2
��SO2ת��ΪSO3 �� ��ŨH2SO4����SO3 ��ش�������⣺
��1������������̢����γɵĹ���ʣ��� ��
��2��SO2������CO2���ƵĻ�ѧ���ʡ������������ʯ������β���е�SO2����д���˷�Ӧ�Ļ�ѧ����ʽ ��
��3���ڢڲ���Ӧ��һ�����Ϸ�Ӧ�����ж�ʵ�ִ˷�Ӧ����һ��Ӧ���� ��
��4����SO2��Cl2ͨ��ˮ����ǡ����ȫ��Ӧ���������ֳ������ᣬ��������л�ѧ����ʽ��Cl2+SO2+2H2O=== +2HCl
��5��ʵ����ϡ��Ũ����ķ����ǣ�
��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 2Fe2O3+8SO2
2Fe2O3+8SO2�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com