
 CaCO3+CO2
CaCO3+CO2 + H2O ��2NaHCO3
+ H2O ��2NaHCO3 Na2CO3+CO2
Na2CO3+CO2 +H2O)�������ɵ�CO2ȫ��ͨ��������ʯ��ˮ�в���bg CaCO3������������С�����ȡ���ȴ����Թ����ּ���������ϡ���ᣬ�ٽ����ɵ�����ȫ��ͨ��������ʯ��ˮ���������c g CaCO3��������ô���й�ϵ��ȷ����
+H2O)�������ɵ�CO2ȫ��ͨ��������ʯ��ˮ�в���bg CaCO3������������С�����ȡ���ȴ����Թ����ּ���������ϡ���ᣬ�ٽ����ɵ�����ȫ��ͨ��������ʯ��ˮ���������c g CaCO3��������ô���й�ϵ��ȷ���� [ ]
 ������ȫ�̼����ĩ���100��ϵ�д�
������ȫ�̼����ĩ���100��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ||
| ||
| A��a=c | B��a��b+c |
| C��b��c | D��a��b |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2009�꽭��ʡ����ѧ��ɳ�����չ����ѧ��ܳ��л�ѧ���������Ծ��������棩 ���ͣ�ѡ����
 CaCO3+CO2��+H2O ��2NaHCO3
CaCO3+CO2��+H2O ��2NaHCO3 Na2CO3+CO2��+H2O ���������ɵ�CO2ȫ��ͨ��������ʯ��ˮ�в���bg CaCO3������������С�����ȡ���ȴ����Թ����ּ���������ϡ���ᣬ�ٽ����ɵ�����ȫ��ͨ��������ʯ��ˮ���������cg CaCO3��������ô���й�ϵһ����ȷ���ǣ� ��
Na2CO3+CO2��+H2O ���������ɵ�CO2ȫ��ͨ��������ʯ��ˮ�в���bg CaCO3������������С�����ȡ���ȴ����Թ����ּ���������ϡ���ᣬ�ٽ����ɵ�����ȫ��ͨ��������ʯ��ˮ���������cg CaCO3��������ô���й�ϵһ����ȷ���ǣ� ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011�꽭��ʡ������������ѧ����ѧУ�п���ѧ����Ծ��������������棩 ���ͣ�ѡ����
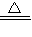 CaCO3+CO2��+H2O ��2NaHCO3
CaCO3+CO2��+H2O ��2NaHCO3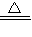 Na2CO3+CO2��+H2O ���������ɵ�CO2ȫ��ͨ��������ʯ��ˮ�в���bg CaCO3������������С�����ȡ���ȴ����Թ����ּ���������ϡ���ᣬ�ٽ����ɵ�����ȫ��ͨ��������ʯ��ˮ���������cg CaCO3��������ô���й�ϵһ����ȷ���ǣ� ��
Na2CO3+CO2��+H2O ���������ɵ�CO2ȫ��ͨ��������ʯ��ˮ�в���bg CaCO3������������С�����ȡ���ȴ����Թ����ּ���������ϡ���ᣬ�ٽ����ɵ�����ȫ��ͨ��������ʯ��ˮ���������cg CaCO3��������ô���й�ϵһ����ȷ���ǣ� ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010�꽭��ʡ�����о�����ѧ�п���ѧ��ģ�Ծ��������棩 ���ͣ�ѡ����
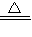 CaCO3+CO2��+H2O ��2NaHCO3
CaCO3+CO2��+H2O ��2NaHCO3 Na2CO3+CO2��+H2O ���������ɵ�CO2ȫ��ͨ��������ʯ��ˮ�в���bg CaCO3������������С�����ȡ���ȴ����Թ����ּ���������ϡ���ᣬ�ٽ����ɵ�����ȫ��ͨ��������ʯ��ˮ���������cg CaCO3��������ô���й�ϵһ����ȷ���ǣ� ��
Na2CO3+CO2��+H2O ���������ɵ�CO2ȫ��ͨ��������ʯ��ˮ�в���bg CaCO3������������С�����ȡ���ȴ����Թ����ּ���������ϡ���ᣬ�ٽ����ɵ�����ȫ��ͨ��������ʯ��ˮ���������cg CaCO3��������ô���й�ϵһ����ȷ���ǣ� ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011����꼶ѧ����¼������ѧѵ���Ծ��������������棩 ���ͣ�ѡ����
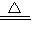 CaCO3+CO2��+H2O ��2NaHCO3
CaCO3+CO2��+H2O ��2NaHCO3 Na2CO3+CO2��+H2O ���������ɵ�CO2ȫ��ͨ��������ʯ��ˮ�в���bg CaCO3������������С�����ȡ���ȴ����Թ����ּ���������ϡ���ᣬ�ٽ����ɵ�����ȫ��ͨ��������ʯ��ˮ���������cg CaCO3��������ô���й�ϵһ����ȷ���ǣ� ��
Na2CO3+CO2��+H2O ���������ɵ�CO2ȫ��ͨ��������ʯ��ˮ�в���bg CaCO3������������С�����ȡ���ȴ����Թ����ּ���������ϡ���ᣬ�ٽ����ɵ�����ȫ��ͨ��������ʯ��ˮ���������cg CaCO3��������ô���й�ϵһ����ȷ���ǣ� ���鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com