
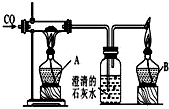
 ČēĶ¼ŹĒÓĆŃõ»ÆĢśĮ¶ĢśŌĄķµÄ×°ÖĆĶ¼£ŗ
ČēĶ¼ŹĒÓĆŃõ»ÆĢśĮ¶ĢśŌĄķµÄ×°ÖĆĶ¼£ŗ
| ||
| ||
| ||
| ||



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
| ||
| ||
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
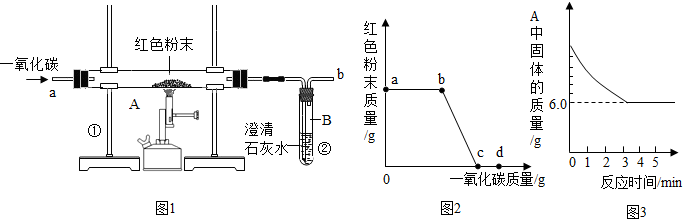
(5·Ö)øÖĢśµÄŅ±Į¶ŹĒČĖĄąĪÄĆ÷µÄŅ»øöÖŲŅŖ±źÖ¾”£Ķ¼1ŹĒŹµŃéŹŅÄ£ÄāĮ¶ĢśµÄ×°ÖĆĶ¼”£


Ķ¼Ņ» Ķ¼¶ž Ķ¼Čż
(1)Š“³öĶ¼1ÖŠA“¦·¢ÉśµÄ»Æѧ·“Ó¦·½³ĢŹ½£ŗ £»
(2)ŹµŃé¹ż³ĢÖŠĶØČėCOÖŹĮæÓėŗģÉ«·ŪÄ©ÖŹĮæµÄ¹ŲĻµČēĶ¼2ĖłŹ¾”£øł¾ŻĶ¼Ź¾£¬ĻĀĮŠĖµ·Ø²»ÕżČ·µÄŹĒ £»£ØĢīŠņŗÅ£©
¢Łaµć±ķŹ¾æŖŹ¼ĶØČėCO£¬·“Ó¦æŖŹ¼·¢Éś
¢Śbµć±ķŹ¾ĶØČėCOŅ»¶ĪŹ±¼äŗó¼ÓČČ£¬·“Ó¦æŖŹ¼·¢Éś
¢Ūcµć±ķŹ¾ŗģÉ«·ŪÄ©ŅŃ³ä·Ö²Ī¼Ó·“Ó¦
¢Üdµć±ķŹ¾·“Ó¦½įŹųŗóČŌŠč¼ĢŠųĶØČėCO
¢ŻæÉøł¾ŻĶØČėCOµÄÖŹĮæ¼ĘĖć³öŗģÉ«·ŪÄ©µÄÖŹĮæ
(3)Ķ¼1×°ÖĆÖŠµÄ²»×ćÖ®“¦ŹĒ ”£
(4)øĆŹµŃéĮ¶ÖʵÄĢśÓė¹¤ŅµĮ¶ÖĘ³öµÄĢś×ī“óµÄĒų±šŹĒ ”£
(5)ij»ÆѧŠ”×éµÄĶ¬Ń§ĆĒĄūÓĆÉĻŹöŹµŃé¶ŌŅ»·Ż¹ĢĢåѳʷ½ųŠŠĮĖĢ½¾æ”£ĶعżŹµŃéŅŃČ·¶ØøĆѳʷÓÉŃõ»ÆĢśŗĶĢś·Ū»ģŗĻ¶ų³É”£ĖūĆĒČ”ĮĖ3.6g¹ĢĢåѳʷ£¬ÓĆĶ¼1ĖłŹ¾µÄ×°ÖĆÖŲŠĀŹµŃ飬²ā¶ØµÄ²æ·ÖŹż¾ŻČēĶ¼3ĖłŹ¾£¬ŌņŌѳʷ֊ĢśŌŖĖŲÓėŃõŌŖĖŲµÄÖŹĮæ±ČŹĒ ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
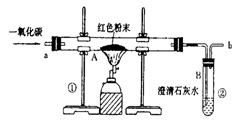
(5·Ö)øÖĢśµÄŅ±Į¶ŹĒČĖĄąĪÄĆ÷µÄŅ»øöÖŲŅŖ±źÖ¾”£Ķ¼1ŹĒŹµŃéŹŅÄ£ÄāĮ¶ĢśµÄ×°ÖĆĶ¼”£



Ķ¼Ņ» Ķ¼¶ž Ķ¼Čż
(1)Š“³öĶ¼1ÖŠA“¦·¢ÉśµÄ»Æѧ·“Ó¦·½³ĢŹ½£ŗ £»
(2)ŹµŃé¹ż³ĢÖŠĶØČėCOÖŹĮæÓėŗģÉ«·ŪÄ©ÖŹĮæµÄ¹ŲĻµČēĶ¼2ĖłŹ¾”£øł¾ŻĶ¼Ź¾£¬ĻĀĮŠĖµ·Ø²»ÕżČ·µÄŹĒ £»£ØĢīŠņŗÅ£©
¢Łaµć±ķŹ¾æŖŹ¼ĶØČėCO£¬·“Ó¦æŖŹ¼·¢Éś
¢Śbµć±ķŹ¾ĶØČėCOŅ»¶ĪŹ±¼äŗó¼ÓČČ£¬·“Ó¦æŖŹ¼·¢Éś
¢Ūcµć±ķŹ¾ŗģÉ«·ŪÄ©ŅŃ³ä·Ö²Ī¼Ó·“Ó¦
¢Üdµć±ķŹ¾·“Ó¦½įŹųŗóČŌŠč¼ĢŠųĶØČėCO
¢ŻæÉøł¾ŻĶØČėCOµÄÖŹĮæ¼ĘĖć³öŗģÉ«·ŪÄ©µÄÖŹĮæ
(3)Ķ¼1×°ÖĆÖŠµÄ²»×ćÖ®“¦ŹĒ ”£
(4)øĆŹµŃéĮ¶ÖʵÄĢśÓė¹¤ŅµĮ¶ÖĘ³öµÄĢś×ī“óµÄĒų±šŹĒ ”£
(5)ij»ÆѧŠ”×éµÄĶ¬Ń§ĆĒĄūÓĆÉĻŹöŹµŃé¶ŌŅ»·Ż¹ĢĢåѳʷ½ųŠŠĮĖĢ½¾æ”£ĶعżŹµŃéŅŃČ·¶ØøĆѳʷÓÉŃõ»ÆĢśŗĶĢś·Ū»ģŗĻ¶ų³É”£ĖūĆĒČ”ĮĖ3.6g¹ĢĢåѳʷ£¬ÓĆĶ¼1ĖłŹ¾µÄ×°ÖĆÖŲŠĀŹµŃ飬²ā¶ØµÄ²æ·ÖŹż¾ŻČēĶ¼3ĖłŹ¾£¬ŌņŌѳʷ֊ĢśŌŖĖŲÓėŃõŌŖĖŲµÄÖŹĮæ±ČŹĒ ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ



²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ2012½ģÖŲĒģŹŠÖŲĒģŅ»ÖŠ¾ÅÄź¼¶ÉĻŃ§ĘŚĘŚÄ©æ¼ŹŌ»ÆѧŹŌ¾ķ£Ø“ų½āĪö£© ĢāŠĶ£ŗĢ½¾æĢā
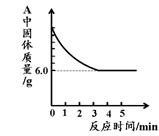
(5·Ö)øÖĢśµÄŅ±Į¶ŹĒČĖĄąĪÄĆ÷µÄŅ»øöÖŲŅŖ±źÖ¾”£Ķ¼1ŹĒŹµŃéŹŅÄ£ÄāĮ¶ĢśµÄ×°ÖĆĶ¼”£

Ķ¼Ņ» Ķ¼¶ž Ķ¼Čż
(1)Š“³öĶ¼1ÖŠA“¦·¢ÉśµÄ»Æѧ·“Ó¦·½³ĢŹ½£ŗ £»
(2)ŹµŃé¹ż³ĢÖŠĶØČėCOÖŹĮæÓėŗģÉ«·ŪÄ©ÖŹĮæµÄ¹ŲĻµČēĶ¼2ĖłŹ¾”£øł¾ŻĶ¼Ź¾£¬ĻĀĮŠĖµ·Ø²»ÕżČ·µÄŹĒ £»£ØĢīŠņŗÅ£©
¢Łaµć±ķŹ¾æŖŹ¼ĶØČėCO£¬·“Ó¦æŖŹ¼·¢Éś
¢Śbµć±ķŹ¾ĶØČėCOŅ»¶ĪŹ±¼äŗó¼ÓČČ£¬·“Ó¦æŖŹ¼·¢Éś
¢Ūcµć±ķŹ¾ŗģÉ«·ŪÄ©ŅŃ³ä·Ö²Ī¼Ó·“Ó¦
¢Üdµć±ķŹ¾·“Ó¦½įŹųŗóČŌŠč¼ĢŠųĶØČėCO
¢ŻæÉøł¾ŻĶØČėCOµÄÖŹĮæ¼ĘĖć³öŗģÉ«·ŪÄ©µÄÖŹĮæ
(3)Ķ¼1×°ÖĆÖŠµÄ²»×ćÖ®“¦ŹĒ ”£
(4)øĆŹµŃéĮ¶ÖʵÄĢśÓė¹¤ŅµĮ¶ÖĘ³öµÄĢś×ī“óµÄĒų±šŹĒ ”£
(5)ij»ÆѧŠ”×éµÄĶ¬Ń§ĆĒĄūÓĆÉĻŹöŹµŃé¶ŌŅ»·Ż¹ĢĢåѳʷ½ųŠŠĮĖĢ½¾æ”£ĶعżŹµŃéŅŃČ·¶ØøĆѳʷÓÉŃõ»ÆĢśŗĶĢś·Ū»ģŗĻ¶ų³É”£ĖūĆĒČ”ĮĖ3.6g¹ĢĢåѳʷ£¬ÓĆĶ¼1ĖłŹ¾µÄ×°ÖĆÖŲŠĀŹµŃ飬²ā¶ØµÄ²æ·ÖŹż¾ŻČēĶ¼3ĖłŹ¾£¬ŌņŌѳʷ֊ĢśŌŖĖŲÓėŃõŌŖĖŲµÄÖŹĮæ±ČŹĒ ”£
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com