
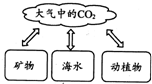
 ��ͼ����Ȼ��̼��ѭ��ʾ��ͼ�����ͼ������ش�
��ͼ����Ȼ��̼��ѭ��ʾ��ͼ�����ͼ������ش�


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
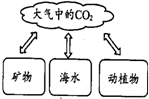 ������̼���������ã���һ����ˮһ����Ҫ��ԭ�ϣ�
������̼���������ã���һ����ˮһ����Ҫ��ԭ�ϣ�
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��2011���Ĵ��㰲��5�⣩��ͼ����Ȼ��̼��ѭ��ʾ��ͼ�����ͼ������ش�
����Ȼ��CO2����Դ;���� ����дһ�㣩��
���κ����ʶ��������ԣ�CO2�����������Ӱ���Ǵٽ�ֲ�������õȣ�����Ӱ����
����дһ�㣩��
�����ཱུ�Ϳ�����CO2�������о�����������һ�Ǽ���CO2�ŷţ���������CO2���ģ���д��һ����������������CO2�ķ�ʽ��;�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
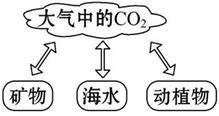 ��ͼ����Ȼ��̼��ѭ��ʾ��ͼ�����ͼ������ش�
��ͼ����Ȼ��̼��ѭ��ʾ��ͼ�����ͼ������ش�
(1)��Ȼ��CO2����Դ;����_________________
(��дһ��)��
(2)�κ����ʶ��������ԣ�CO2�����������Ӱ���Ǵٽ�ֲ�������õȣ�����Ӱ����_____________________________________________________(��дһ��)��
(3)���ཱུ�Ϳ�����CO2�������о�����������һ�Ǽ���CO2�ŷţ���������CO2���ģ���д��һ����������������CO2�ķ�ʽ��;��___________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011��ȫ���п���ѧ����������13 ������̼��ȡ������ ���ͣ������
��2011���Ĵ��㰲��5�⣩��ͼ����Ȼ��̼��ѭ��ʾ��ͼ�����ͼ������ش�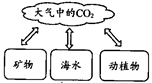
����Ȼ��CO2����Դ;���� ����дһ�㣩��
���κ����ʶ��������ԣ�CO2�����������Ӱ���Ǵٽ�ֲ�������õȣ�����Ӱ����
����дһ�㣩��
�����ཱུ�Ϳ�����CO2�������о�����������һ�Ǽ���CO2�ŷţ���������CO2���ģ���д��һ����������������CO2�ķ�ʽ��;�� ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com