
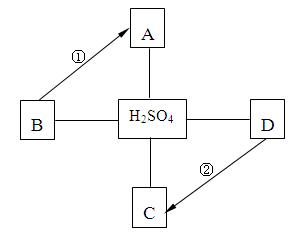
A~D�������Ѿ�ѧ�������ֲ�ͬ���ij������ʣ�����B�Ǻ�ɫ���壬 C�����ڿ������׳��⣬A��B��C��D��H2SO4֮������ϵ����ͼ��ʾ�� ��������ʾ���ʼ��ת����ϵ�� ����������ʾ���ʼ�������Ӧ������ش��������⣺

��1��д���������ʵĻ�ѧʽ��B ��C ��
��2�������ʷ����У�C���� ����ᡱ����������Ρ������������
��3���ڵĻ�ѧ����ʽ�� ���÷�Ӧ�Ļ��������� ��
��4��������һ���û���Ӧ����Ӧ�Ļ�ѧ����ʽ�� ��
��5����д�������������ʷ�Ӧ��һ������ ��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��9�֣���A~D�������Ѿ�ѧ�������ֳ������ʣ�����A�Ǻ�ɫ���壬 D���ڲ�������ֽ����֯��ϴ�Ӽ��������� A��B��C��D������ͼ������ϡH2SO4��Ca(OH)2֮������ϵ����ͼ��ʾ�� ��������ʾ���ʼ��ת����ϵ������������ʾ���ʼ�������Ӧ������ش��������⣺

��1��H2SO4�� Ca(OH)2��Ӧ�Ļ��������� ��
��2��д���������ʵĻ�ѧʽ��B ��C ��
��3��д�����з�Ӧ�Ļ�ѧ����ʽ���� ���� ��
��4��������һ���û���Ӧ����Ӧ�Ļ�ѧ����ʽ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012��㶫ʡ��ͷ�κ����п�ģ�⿼��ѧ�� ���������� ���ͣ��ƶ���
��9�֣���A~D�������Ѿ�ѧ�������ֳ������ʣ�����A�Ǻ�ɫ���壬 D���ڲ�������ֽ����֯��ϴ�Ӽ��������� A��B��C��D������ͼ������ϡH2SO4��Ca(OH)2֮������ϵ����ͼ��ʾ�� ��������ʾ���ʼ��ת����ϵ������������ʾ���ʼ�������Ӧ������ش��������⣺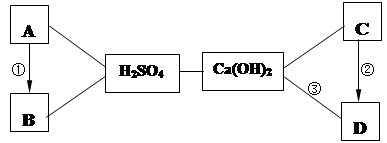
��1��H2SO4�� Ca(OH)2��Ӧ�Ļ��������� ��
��2��д���������ʵĻ�ѧʽ��B ��C ��
��3��д�����з�Ӧ�Ļ�ѧ����ʽ���� ���� ��
��4��������һ���û���Ӧ����Ӧ�Ļ�ѧ����ʽ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
A~D�������Ѿ�ѧ�������ֳ������ʣ�����A�Ǻ�ɫ���壬 D���ڲ�������ֽ����֯��ϴ�Ӽ��������� A��B��C��D������ͼ������ϡH2SO4��Ca(OH)2֮������ϵ����ͼ��ʾ�� ��������ʾ���ʼ��ת����ϵ����——����ʾ���ʼ�������Ӧ������ش��������⣺
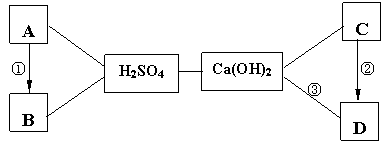 |
��1��H2SO4�� Ca(OH)2��Ӧ�Ļ��������� ��
��2��д���������ʵĻ�ѧʽ��B ��C ��
��3��д�����з�Ӧ�Ļ�ѧ����ʽ���� ���� ��
��4��������һ���û���Ӧ����Ӧ�Ļ�ѧ����ʽ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
A~D�������Ѿ�ѧ�������ֳ������ʣ�����A�Ǻ�ɫ���壬 D���ڲ�������ֽ����֯��ϴ�Ӽ��������� A��B��C��D������ͼ������ϡH2SO4��Ca(OH)2֮������ϵ����ͼ��ʾ�� ��������ʾ���ʼ��ת����ϵ����——����ʾ���ʼ�������Ӧ������ش������� �⣺
�⣺
 |
��1��H2SO4�� Ca(OH)2��Ӧ�Ļ��������� ��
��2��д���������ʵĻ�ѧʽ��B ��C ��
��3��д�����з�Ӧ�Ļ�ѧ����ʽ���� ���� ��
��4��������һ���û���Ӧ����Ӧ�Ļ�ѧ����ʽ�� ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com