
| С���ϣ� �Ȼ�����Һ��PHΪ7�� NaCO3+CaCl2=2NaCl+CaCO3�� |
| 106 |
| 75g��21.2% |
| 117 |
| x |


 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
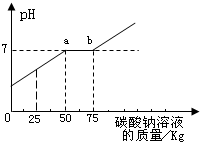
| С���ϣ� �Ȼ�����Һ��PHΪ7�� NaCO3+CaCl2=2NaCl+CaCO3�� 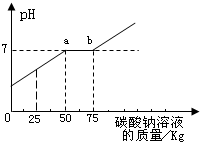 ��1��ͨ��ͼ������Ͱ�еķ�Һ�ﺬ�е������� ��2��ͨ��ͼ��֪����̼������Һ�����ӵ� ��3��ͼ�б�ʾa��ĺ��� ��4������������Һ���Ȼ��Ƶ�����Ϊ �鿴�𰸺ͽ���>> ��Ŀ�����л�ѧ ��Դ�� ���ͣ� 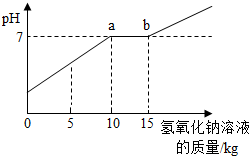 ��2009?���ţ�ͨ����ʵ�������ꡰ��������ȡ��ʵ���Ҫ����Һ�����ҺͰ�У������Ϳ��ռ�һЩ���������п�Ļ����Һ���������������ʣ���Ϊ������Ⱦ������ijУ��ѧ��ȤС���ͬѧ��������ʵ�飺 ��2009?���ţ�ͨ����ʵ�������ꡰ��������ȡ��ʵ���Ҫ����Һ�����ҺͰ�У������Ϳ��ռ�һЩ���������п�Ļ����Һ���������������ʣ���Ϊ������Ⱦ������ijУ��ѧ��ȤС���ͬѧ��������ʵ�飺ȡ��ҺͰ�ϲ���Һ��12.2kg�������м���������������Ϊ8%������������Һ��������ҺpH����������������Һ��������ϵ��ͼ��ʾ������֪ZnSO4+2NaOH�TZn��OH��2��+Na2SO4��ZnSO4��Һ��pH�ӽ�7�� ��1��ͼ�б�ʾ��a��ĺ����ǣ� ��ʾ��������������ǡ����ȫ��Ӧ ��ʾ��������������ǡ����ȫ��Ӧ ����2��ͨ��ͼ��֪��������������Һ�������ӵ� 15 15 kgʱ����Һǡ�ô����꣮�鿴�𰸺ͽ���>> ��Ŀ�����л�ѧ ��Դ�� ���ͣ������ ������ʵ�������ꡰ������̼����ȡ��ʵ�����Һ����Ͱ���ռ��˴����Ļ����Һ�����ǹ������ʣ���Ϊ������Ⱦ��������ѧ��ȤС����������ʵ�飺
�鿴�𰸺ͽ���>> ��Ŀ�����л�ѧ ��Դ��2009�걱���в�ƽ���п���ѧһģ�Ծ��������棩 ���ͣ������ ��2009?��ƽ��һģ��������ʵ�������ꡰ������̼����ȡ��ʵ�����Һ����Ͱ���ռ��˴����Ļ����Һ�����ǹ������ʣ���Ϊ������Ⱦ��������ѧ��ȤС����������ʵ�飺 ȡ��ҺͰ�ϲ���Һ��59.4kg�������м���������������Ϊ21.2%��̼������Һ��������ҺpH������̼������Һ��������ϵ��ͼ��ʾ��
 ��1��ͨ��ͼ������Ͱ�еķ�Һ�ﺬ�е������ǣ� ��2��ͨ��ͼ��֪����̼������Һ�����ӵ�kgʱ����Һǡ�ô����꣮ ��3��ͼ�б�ʾa��ĺ��壮 ��4������������Һ���Ȼ��Ƶ�����Ϊ�� �鿴�𰸺ͽ���>> ͬ����ϰ��� ����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר�� Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com��Ȩ��������վ�������£�ͼƬ��Դ�����磬����Ȩ����Ȩ��ԭ�������У�ת�������ַ���Ȩ��������Ȩ����������������֪�����ǽ����촦������ϵqq��3310059649�� ICP�������: ��ICP��07509807��-10 ����������42018502000812�� |