
 Na2CO3+CO2��+H2O��ij������Ʒ�л���������̼�����ƣ�Ϊ�˲ⶨ��Ʒ�Ĵ��ȣ���ѩͬѧ��������ʵ�飺ȷ��ȡ��Ʒ10.0g�����Թ��У����ȳ�ַ�Ӧ��������ų���������CO2����224mL����������������ܶ�Ϊ1.964g/L������ش��������⣺
Na2CO3+CO2��+H2O��ij������Ʒ�л���������̼�����ƣ�Ϊ�˲ⶨ��Ʒ�Ĵ��ȣ���ѩͬѧ��������ʵ�飺ȷ��ȡ��Ʒ10.0g�����Թ��У����ȳ�ַ�Ӧ��������ų���������CO2����224mL����������������ܶ�Ϊ1.964g/L������ش��������⣺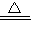 Na2CO3+H2O+CO2��
Na2CO3+H2O+CO2�� 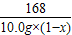 =
=
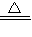 Na2CO3+H2O+CO2�� ���ٵ�����
Na2CO3+H2O+CO2�� ���ٵ�����  =
=
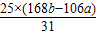 %��
%��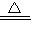 Na2CO3+H2O+CO2�� ���ٵ�����
Na2CO3+H2O+CO2�� ���ٵ����� 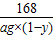 =
=
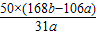 %
%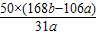 %��
%��

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012��ɽ��ʡ���������������꼶�п�һģ��ѧ�Ծ����������� ���ͣ�̽����
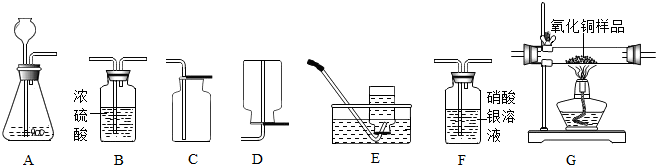
��6�֣���ͼΪʵ�����г����������Ʊ����������ռ�������ʵ��IJ����������Ը�����ĿҪ�ش��������⣺ A B C D E F G
A B C D E F G
������ʯ��ʯ��ϡ����Ϊԭ����ȡ������̼���壬�������ӷ����������Ȼ������壬������ȡ�����岻������Ҫ�ռ�һƿ��������Ķ�����̼���塣
����ѡ����������˳��Ϊ ����д���������ĸ����
�����ɶ�����̼ʱ����������Ӧ�Ļ�ѧ����ʽΪ�� ��
���������F�г��� ������֤�������л����Ȼ������塣
��ʵ������ȡ������̼���ʣ���Һ�У�������ʣ����������ᣬ����֤����Ĵ��ڣ����AgNO3��Һ��ʯ�Na2CO3��Һ�����Լ���ѡ��һ���Լ�������֤��������ѡ������� ��
��������A��ʢ��Zn��H2SO4��Һ��ijͬѧ�������Ʊ����������ⶨCuO��Ʒ��CuO�Ĵ��ȣ����ʲ���Ӧ��������������˳��ΪA��G��B������֪��CuO+H2 �� Cu+H2O��
��ʵ��ʱ����۲쵽װ��G�еĺ�ɫ������ ɫ��
�ڸ�ͬѧͨ��������Ӧǰ��Bװ�����������ӣ���������Ʒ��CuO�Ĵ��ȣ���������� ���ƫ����ƫС����������Ӱ�족֮һ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ��ɽ��ʡ���������������꼶�п�һģ��ѧ�Ծ��������棩 ���ͣ�̽����
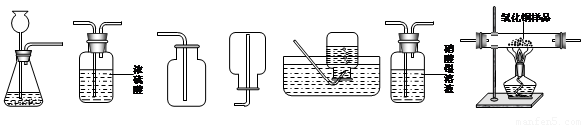
��6�֣���ͼΪʵ�����г����������Ʊ����������ռ�������ʵ��IJ����������Ը�����ĿҪ�ش��������⣺
 A
B
C
D
E
F G
A
B
C
D
E
F G
������ʯ��ʯ��ϡ����Ϊԭ����ȡ������̼���壬�������ӷ����������Ȼ������壬������ȡ�����岻������Ҫ�ռ�һƿ��������Ķ�����̼���塣
����ѡ����������˳��Ϊ ����д���������ĸ����
�����ɶ�����̼ʱ����������Ӧ�Ļ�ѧ����ʽΪ�� ��
���������F�г��� ������֤�������л����Ȼ������塣
��ʵ������ȡ������̼���ʣ���Һ�У�������ʣ����������ᣬ����֤����Ĵ��ڣ����AgNO3��Һ��ʯ�Na2CO3��Һ�����Լ���ѡ��һ���Լ�������֤��������ѡ������� ��
��������A��ʢ��Zn��H2SO4��Һ��ijͬѧ�������Ʊ����������ⶨCuO��Ʒ��CuO�Ĵ��ȣ����ʲ���Ӧ��������������˳��ΪA��G��B������֪��CuO+H2 �� Cu+H2O��
��ʵ��ʱ����۲쵽װ��G�еĺ�ɫ������ ɫ��
�ڸ�ͬѧͨ��������Ӧǰ��Bװ�����������ӣ���������Ʒ��CuO�Ĵ��ȣ���������� ���ƫ����ƫС����������Ӱ�족֮һ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
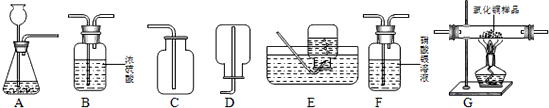
 Cu+H2O��
Cu+H2O���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012��ɽ��ʡ�������������п���ѧһģ�Ծ��������棩 ���ͣ������
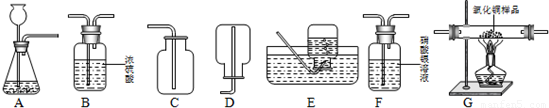
 Cu+H2O��
Cu+H2O���鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com