


 ��ǰ�κ�ͬ����ϰϵ�д�
��ǰ�κ�ͬ����ϰϵ�д� ����С��ҵϵ�д�
����С��ҵϵ�д� �Ƹ�С״Ԫ����������ϰ��ϵ�д�
�Ƹ�С״Ԫ����������ϰ��ϵ�д� �ɹ�ѵ���ƻ�ϵ�д�
�ɹ�ѵ���ƻ�ϵ�д� ����ѵ����ֱͨ�п�����ϵ�д�
����ѵ����ֱͨ�п�����ϵ�д� һ���㶨ϵ�д�
һ���㶨ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��2006?���ϣ��ᾧ�Ƿ�������Թ��������һ�ֳ��÷������ҹ������������κ�����ˮ�����д�����̼���ƺ��Ȼ��ƣ������ũ�����̡����̼���ƣ�������ɹ�Σ��Ȼ��ƣ��������ж�ͼ���ܷ�ӳ̼�����ܽ�ȱ仯�������ǣ�������
��2006?���ϣ��ᾧ�Ƿ�������Թ��������һ�ֳ��÷������ҹ������������κ�����ˮ�����д�����̼���ƺ��Ȼ��ƣ������ũ�����̡����̼���ƣ�������ɹ�Σ��Ȼ��ƣ��������ж�ͼ���ܷ�ӳ̼�����ܽ�ȱ仯�������ǣ��������鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
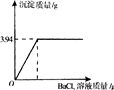 ��2006?���ϣ�ʵ��������һƿ�����ռ��������˿����еĶ�����̼�����ֱ��ʣ�Ϊ�ⶨ��ƿ�ռ�Ĵ��ȣ���ȡ����Ʒ10g����ˮ�����Һ����������μ����Ȼ�����Һ��������ȫ����Ӧ����ʽΪNa2CO3+BaCl2�TBaCO3��+2NaCl������Ӧ���������ɳ����������������Ȼ�����Һ�����Ĺ�ϵ����ͼ��ʾ���������ռ���Ʒ���������Ƶ�����������
��2006?���ϣ�ʵ��������һƿ�����ռ��������˿����еĶ�����̼�����ֱ��ʣ�Ϊ�ⶨ��ƿ�ռ�Ĵ��ȣ���ȡ����Ʒ10g����ˮ�����Һ����������μ����Ȼ�����Һ��������ȫ����Ӧ����ʽΪNa2CO3+BaCl2�TBaCO3��+2NaCl������Ӧ���������ɳ����������������Ȼ�����Һ�����Ĺ�ϵ����ͼ��ʾ���������ռ���Ʒ���������Ƶ������������鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com