
���� ��1���������ʵ�����=��Һ�����������ʵ������������з�����
��2������Ũ��Һϡ��Ϊϡ��Һ�����ʵ��������䣬ѡ����Ͳʱѡȡ������ȡҺ�������������Ͳ������һ������ȡ����С�����з�����
��3�������������ƹ�����ǿ�ҵĸ�ʴ�ԣ���Ͳֻ����ȡҺ�����������ƺõ���Һ��ǩװƿ���ݴ˷������
��4���������ƺ���Һ���ʵ�������������10%�����ܵ�ԭ�����ܼ����˻��������˽��з�����
��� �⣺��1������50g��������Ϊ10%������������Һ�������������ƹ������ƣ����ȡ��������50g��10%=5g��
��2��Ũ��Һϡ��Ϊϡ��Һ�����ʵ��������䣬����Ҫ25%������������Һ������Ϊx������5g=x��25%�����x=20g��������Ҫ����ˮ������Ϊ50g-20g=30g��ˮ���ܶ�Ϊ1g/cm3����Ҫˮ30mL��������Ͳѡȡ��һ���������ԭ��Ӧѡ50mL����Ͳ��
��3��A���������ƹ�����ǿ�ҵĸ�ʴ�ԣ�Ӧ���ڲ��������г�������ֹ��ʴ��ƽ���̣���A����
B����Ͳֻ����ȡҺ��������������Ϊ������Һ����������B����
C�����ƺõ���Һ��ǩװƿ����C��ȷ��
��ѡ��C��
��4�����ƺ���Һ���ʵ�������������10%�����ܵ�ԭ�����ܼ����˻��������ˣ�����Ϊ���ܵ�ԭ�������Ӷ�����
�ʴ�Ϊ����1��5��
��2��20��50��
��3��C��
��4�����Ӷ�����
���� �������ʵ����������ļ���ʽ�������Ӧ�ý��������⣬�˽�Ũ��Һ����ϡ��Һ�ķ�������Һ���Ƶ�ע��������ǽ������Ĺؼ����أ�


 �ִʾ�ƪ��ͬ�����Ĵ��ϵ�д�
�ִʾ�ƪ��ͬ�����Ĵ��ϵ�д� �߽�������ϵ�д�
�߽�������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 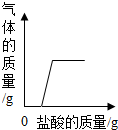 ���ֱ��ʵ�NaOH��Һ�еμ�ϡ���� ���ֱ��ʵ�NaOH��Һ�еμ�ϡ���� | |
| B�� | 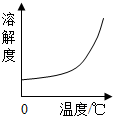 HCl������ܽ�����¶�Ӱ��ı仯���� HCl������ܽ�����¶�Ӱ��ı仯���� | |
| C�� | 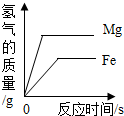 ��������Mg�ۺ�Fe����������ͬŨ�ȵ�ϡ���ᷴӦ ��������Mg�ۺ�Fe����������ͬŨ�ȵ�ϡ���ᷴӦ | |
| D�� | 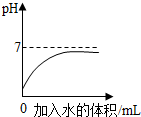 ������Һϡ������pH�ı仯���� ������Һϡ������pH�ı仯���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
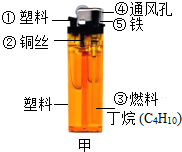 ȼ���ڹ�ũҵ�������ճ����������Ź㷺�����ã�
ȼ���ڹ�ũҵ�������ճ����������Ź㷺�����ã�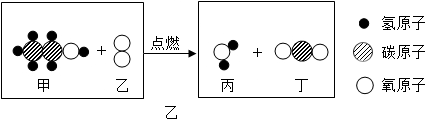
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ���п��������ձ��У�δ���������Ⱦָ����Ŀ����PM10 | |
| B�� | ������̼�ڿ����к����������������ЧӦ�����ڿ�����Ⱦ | |
| C�� | ��ú̿�м���ʯ��ʯ����ʯ��������������Լ��ٶ���������ŷ� | |
| D�� | ���������ﳾ�Ķ��ٲ�Ӱ�����彡�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� |  �ں��µ������£����Ȼ��Ʋ�������Һ����������ˮ�� �ں��µ������£����Ȼ��Ʋ�������Һ����������ˮ�� | |
| B�� | 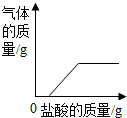 ������̶Ƚϴ�������м������ϡ���� ������̶Ƚϴ�������м������ϡ���� | |
| C�� | 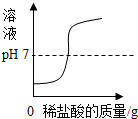 ������������Һ�в��ϵμ�ϡ���� ������������Һ�в��ϵμ�ϡ���� | |
| D�� | 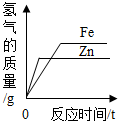 ��ͬ�������ҹ�����п�ۺ����ۣ��ֱ�������������������ͬ��ϡ���ᷴӦ ��ͬ�������ҹ�����п�ۺ����ۣ��ֱ�������������������ͬ��ϡ���ᷴӦ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
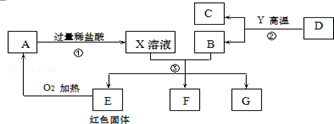
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ������ֻ��̼����Ԫ�� | |
| B�� | ������һ����̼����Ԫ�أ����ܺ�����Ԫ�� | |
| C�� | ��������̼���⡢��Ԫ����� | |
| D�� | �����ʷ�����̼ԭ�Ӻ���ԭ�ӵĸ�����Ϊ1��1 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ���촵������--������������Ż�� | |
| B�� | ����ɭ�ֻ���ʱ���ٸ����--��������ȼ�� | |
| C�� | ��������ú¯��Խ��Խ��--����ȼ����������� | |
| D�� | ʵ���Ҿƾ��Ƶ�Ϩ���õ�ñ����--�������� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com