
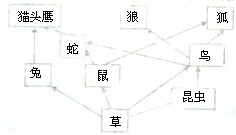
 ��ͼ�Ǵ���ƽ��״̬��ij�´���ԭ��̬ϵͳ��ʳ��ͼ����ش�
��ͼ�Ǵ���ƽ��״̬��ij�´���ԭ��̬ϵͳ��ʳ��ͼ����ش�| ������ | A | B | C | D | E | ||||||||||||||||||||
| �ؽ���Ũ�ȣ�ppm�� | 0.05 | 7 | 0.51 | 68 | 0.29��5����ͼֻ��ʾ������̬ϵͳ�IJ��ֳɷ֣�����Ϊͼ��δ��ʾ��������̬ϵͳ�ijɷֻ��� ��6�������ڸòݳ�����ţ��Ϊ�����������������ţ������ڣ��������������Ĺ��ɺ��ص㣬����Ϊ����Ӧ����ô����������д��һ�㽨�飩 ��7��Ϊ�����Ӿ������룬�����ڸòݳ����ȷ���������֮�ݵ����ػ�Į�����ݵ���������ή����Ϊ��������Į����������������Ǩ���õ������ظ���ԭ����Ȼ״̬�������ԭ���·�ʢ��������̬�ָ�ƽ�⣬��˵����̬ϵͳ����һ���� ��������һ���Ŀռ䷶Χ�ڣ������뻷�����γɵ�ͳһ�����������̬ϵͳ����̬ϵͳ����ɰ��������ﲿ�ֺ����ﲿ�֣���̬ϵͳ�����ҵ�����������һ���ȵģ�ͨ�������̬ϵͳ����ɺ����ֵ����ý��н�� ����⣺��1����̬ϵͳ�е����ʺ���������ʳ���������ģ��������������ĺ������ģ��Լ�ֲ��IJ�֦��Ҷ�Ͷ���Ĺ�����Ƥë�����Ա���һ��Ӫ�������������ã����������������ʳ���������Ĺ��������ݼ��ģ�һ����˵�������뵽һ��Ӫ�����������У�ֻ��10%��20%�������ܹ�������һ��Ӫ���������ԣ���ʳ������Ӫ����Խ�࣬������ʧ��Խ�࣬���Ӫ��������õ�������Խ�٣����������������Խ�٣�Ӫ����Խ�ٵ�ʳ���������Ӫ�������������Խ�࣬ͼ�е�ʳ�����вݡ��á�èͷӥ��Ӫ�������٣�èͷӥ��õ�������࣮ ��2������̬ϵͳ�У���������֮������ʳ���ϵ���γɵ�һ����ϵ������ʳ��������ֲ��֮�����ͨ��ʳ�������ϵ�����ģ���ֲ��֮���dz��뱻�ԵĹ�ϵ��ʳ�����ɱ�ʾΪ�������ߡ����������ߡ��μ������ߡ����������ߵȵȣ�ͼ�й���8��ʳ�������ֱ��ǣ��ݡ��á�èͷӥ���ݡ�����ߡ�èͷӥ���ݡ���������ݡ���������ݡ�����ǡ��ݡ���������èͷӥ���ݡ����������ǡ��ݡ����������������е�ʳ��������λ�еڶ�Ӫ�����������У��������桢�ã� ��3������ͬ��2������̬ϵͳ�У���������֮������ʳ���ϵ���γɵ�һ����ϵ������ʳ��������ֲ��֮�����ͨ��ʳ�������ϵ�����ģ���ֲ��֮���dz��뱻�ԵĹ�ϵ��ʳ�����ɱ�ʾΪ�������ߡ����������ߡ��μ������ߡ����������ߵȵȣ�ͼ�й���8��ʳ�������ֱ��ǣ��ݡ��á�èͷӥ���ݡ�����ߡ�èͷӥ���ݡ���������ݡ���������ݡ�����ǡ��ݡ���������èͷӥ���ݡ����������ǡ��ݡ������������� ��4������̬ϵͳ���ж����ʣ��ؽ����ȣ���ʳ����������Ӫ������������и�������Ӫ������Խ�������۵��ж�����Խ�ߣ��ڸ���̬ϵͳ�����Ӫ������������������۵��ж�������࣮���ݱ���ABCDE������������Ⱦ���ж����ʺ����Ķ��٣���ɵ�ʳ�����ǣ�A��E��C��B��D������λ�ڵ���Ӫ������������C�� ��5����̬ϵͳ���ɷ�����ɷֺ�����ɷ���������ɵģ�����ɷְ�����̬ϵͳ�е�ȫ��������ݻ�õ�Ӫ���������ķ�ʽ������ɷ��ֿ��Ի���Ϊ�����ߡ������ߡ��ֽ��ߣ��ڸ���̬ϵͳ�У�ȱ�ٵĻ��з�����ɷ�����ͷֽ��ߣ� ��6�������ڸòݳ�����ţ��Ϊ�����������������ţ������ڣ��������������Ĺ��ɺ��ص㣬�ʵ���ɱһЩ�ú����� ��7����̬ϵͳ�и�������������������������ȶ���״̬������̬ƽ�⣬Ϊ�����Ӿ������룬�����ڸòݳ����ȷ���������֮�ݵ����ػ�Į�����ݵ���������ή����Ϊ��������Į����������������Ǩ���õ������ظ���ԭ����Ȼ״̬�������ԭ���·�ʢ��������̬�ָ�ƽ�⣮˵����̬ϵͳ����һ�������ҵ�����������������������һ���ȵģ� �ʴ�Ϊ����1���ݡ��á�èͷӥ ��2���������桢�� ��3��5 ��4��C ��5��������ɷ� �ֽ��� ��6���ʵ���ɱһЩ�ú�����7�����ҵ������� ���������⿼��ѧ������̬ϵͳ����ɣ�ʳ��������̬ƽ�����̬ϵͳ�����ҵ��ڼ����︻����֪ʶ����������������ѧ���ķ��������������������������һ�����ۺ��ԣ��ѶȽϴ�  
��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д� ��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д� Сѧ��10����Ӧ����ϵ�д�
���ϰ��
��Ŀ���������� ��Դ�� ���ͣ�  ��2010?������ͼ�Ǵ���ƽ��״̬��ij��ԭ��̬ϵͳ��ʳ�����������ͼ�����ش��������⣺ ��2010?������ͼ�Ǵ���ƽ��״̬��ij��ԭ��̬ϵͳ��ʳ�����������ͼ�����ش��������⣺��1�����ʳ������ 4 4 ��ʳ�������ɣ���2���˲�ԭ�е��������P���������ͬ���� ��̬ϵͳ ��̬ϵͳ ����3����èͷӥ���ԣ������������ٵ�ʳ������ ��ɫֲ������èͷӥ ��ɫֲ������èͷӥ ����4��èͷӥ���ڵ��л�����������Դ�� ��ɫֲ�� ��ɫֲ�� ���е���� ��� ���ã���5������������˿��칤�������¸���̬ϵͳ�������ؽ�����Ⱦ����ͼ�� èͷӥ èͷӥ �ڵ��ؽ���������ߣ��鿴�𰸺ͽ���>> ��Ŀ���������� ��Դ�� ���ͣ�  ��ͼ�Ǵ���ƽ��״̬��ij�´���ԭ��̬ϵͳ��ʳ������ ��ͼ�Ǵ���ƽ��״̬��ij�´���ԭ��̬ϵͳ��ʳ��������1��������������Χ���Ǻͺ��������ԭֲ���ܵ��ƻ�����ԭ���� ʳ������ƻ� ʳ������ƻ� ����2��ֹͣΧ�Լ�����Ǻͺ����´�����ֳ����̬�ָ�ƽ�⣮��˵����̬ϵͳ���� �Զ����� �Զ����� ������һ���˵����̬ϵͳ�ijɷ�Խ�� �� ��Ӫ���ṹԽ���� ���� ���Զ����� �Զ����� ����Խǿ����̬ƽ��Խ�������� ���� �����ǣ����������������һ���ȣ���̬ƽ�����ʧȥƽ�� ʧȥƽ�� ���鿴�𰸺ͽ���>> ��Ŀ���������� ��Դ�� ���ͣ�  ��ͼ�Ǵ���ƽ��״̬��ij��ԭ��̬ϵͳ��ʳ�����������ͼ�����ش��������⣺ ��ͼ�Ǵ���ƽ��״̬��ij��ԭ��̬ϵͳ��ʳ�����������ͼ�����ش��������⣺��1�����ʳ������ 4 4 ��ʳ�������ɣ���2���˲�ԭ�е��������P���������ͬ���� ��̬ϵͳ ��̬ϵͳ ����3����èͷӥ���ԣ������������ٵ�ʳ������ ��ɫֲ������èͷӥ ��ɫֲ������èͷӥ ����4��èͷӥ���ڵ��л�����������Դ����ɫֲ����е� ��� ��� ���ã��鿴�𰸺ͽ���>> ��Ŀ���������� ��Դ�� ���ͣ�  ��ͼ�Ǵ���ƽ��״̬��ij�ȴ���ԭ��̬ϵͳ��ʳ��������ش� ��ͼ�Ǵ���ƽ��״̬��ij�ȴ���ԭ��̬ϵͳ��ʳ��������ش���1���������̬ϵͳ��ʳ�����а�������ʳ�������ֱ���ʲô�� ��2����������������Χ�Ժ��������ԭֲ���ܵ��ƻ�����ԭ����ʲô�� ��3��ֹͣΧ�ԣ����������������࣬��̬ϵͳ�ָ�ƽ�⣮��˵����̬ϵͳ���� һ�����Զ����� һ�����Զ����� �������鿴�𰸺ͽ���>> ͬ����ϰ��� ����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר�� Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com��Ȩ��������վ�������£�ͼƬ��Դ�����磬����Ȩ����Ȩ��ԭ�������У�ת�������ַ���Ȩ��������Ȩ����������������֪�����ǽ����촦������ϵqq��3310059649�� ICP�������: ��ICP��07509807��-10 ����������42018502000812�� | ||||||||||||||||||||