
”¾ĢāÄæ”æĪŹĢāĢ½¾æ
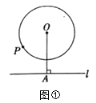
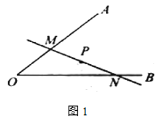
£Ø1£©ČēĶ¼¢Ł£¬ŅŃÖŖ![]() ÓėÖ±Ļß
ÓėÖ±Ļß![]() £¬¹ż
£¬¹ż![]() ×÷
×÷![]() ÓŚµć
ÓŚµć![]() £¬
£¬![]() £¬
£¬![]() µÄ°ė¾¶ĪŖ
µÄ°ė¾¶ĪŖ![]() £¬ŌņŌ²ÉĻŅ»µć
£¬ŌņŌ²ÉĻŅ»µć![]() µ½
µ½![]() µÄ¾ąĄėµÄ×īŠ”ÖµŹĒ______£»
µÄ¾ąĄėµÄ×īŠ”ÖµŹĒ______£»
 ””””””
”””””” ””””
””””
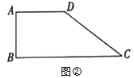
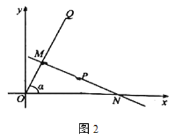
£Ø2£©ČēĶ¼¢Ś£¬ŌŚĖıߊĪ![]() ÖŠ£¬
ÖŠ£¬![]() £¬
£¬![]() £¬
£¬![]() £¬
£¬![]() £¬¹żµć
£¬¹żµć![]() ×÷Ņ»ĢõÖ±Ļß½»±ß
×÷Ņ»ĢõÖ±Ļß½»±ß![]() »ņ
»ņ![]() ÓŚ
ÓŚ![]() £¬Čō
£¬Čō![]() Ę½·ÖĖıߊĪ
Ę½·ÖĖıߊĪ![]() µÄĆ껿£¬Ēó
µÄĆ껿£¬Ēó![]() µÄ³¤£»
µÄ³¤£»
ĪŹĢā½ā¾ö
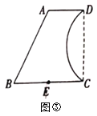
£Ø3£©ČēĶ¼¢ŪĖłŹ¾£¬ŹĒÓÉĻ߶Ī![]() ”¢
Ӣ![]() Ӣ
”¢![]() Óė»”
Óė»”![]() Ī§³ÉµÄ»ØŌ°µÄĘ½ĆęŹ¾ŅāĶ¼£¬
Ī§³ÉµÄ»ØŌ°µÄĘ½ĆęŹ¾ŅāĶ¼£¬![]() £¬
£¬![]() £¬
£¬![]() //
//![]() £¬CD”ĶBC£¬µć
£¬CD”ĶBC£¬µć![]() ĪŖ
ĪŖ![]() µÄÖŠµć£¬
µÄÖŠµć£¬![]() Ėł¶ŌµÄŌ²ŠÄ½ĒĪŖ
Ėł¶ŌµÄŌ²ŠÄ½ĒĪŖ![]() £®¹ÜĄķČĖŌ±ĻėŌŚ
£®¹ÜĄķČĖŌ±ĻėŌŚ![]() ÉĻČ·¶ØŅ»µć
ÉĻČ·¶ØŅ»µć![]() £¬ŌŚĖıߊĪ
£¬ŌŚĖıߊĪ![]() ĒųÓņÖÖÖ²»Ø»Ü£¬ĘäÓąĒųÓņÖÖÖ²²ŻĘŗ£¬²¢¹ż
ĒųÓņÖÖÖ²»Ø»Ü£¬ĘäÓąĒųÓņÖÖÖ²²ŻĘŗ£¬²¢¹ż![]() µćŠŽ½ØŅ»ĢõŠ”Ā·
µćŠŽ½ØŅ»ĢõŠ”Ā·![]() £¬°ŃĖıߊĪ
£¬°ŃĖıߊĪ![]() ·Ö³ÉĆ껿ĻąµČĒŅ¾”æÉÄÜŠ”µÄĮ½²æ·Ö£¬·Ö±šÖÖÖ²²»Ķ¬µÄ»Ø»Ü£®ĪŹŹĒ·ń“ęŌŚĀś×ćÉĻŹöĢõ¼žµÄŠ”Ā·
·Ö³ÉĆ껿ĻąµČĒŅ¾”æÉÄÜŠ”µÄĮ½²æ·Ö£¬·Ö±šÖÖÖ²²»Ķ¬µÄ»Ø»Ü£®ĪŹŹĒ·ń“ęŌŚĀś×ćÉĻŹöĢõ¼žµÄŠ”Ā·![]() £æČō“ęŌŚ£¬ĒėĒó³ö
£æČō“ęŌŚ£¬ĒėĒó³ö![]() µÄ³¤£¬Čō²»“ęŌŚ£¬ĒėĖµĆ÷ĄķÓÉ£®
µÄ³¤£¬Čō²»“ęŌŚ£¬ĒėĖµĆ÷ĄķÓÉ£®
”¾“š°ø”æ£Ø1£©![]() £»£Ø2£©
£»£Ø2£©![]() £»£Ø3£©“ęŌŚĀś×ćÉĻŹöĢõ¼žµÄŠ”Ā·
£»£Ø3£©“ęŌŚĀś×ćÉĻŹöĢõ¼žµÄŠ”Ā·![]() £¬
£¬![]() µÄ³¤ĪŖ
µÄ³¤ĪŖ![]() £®
£®
”¾½āĪö”æ
£Ø1£©Ō²ÉĻŅ»µć![]() µ½
µ½![]() µÄ¾ąĄėµÄ×īŠ”Öµ¼“ŹĒŌ²ŠÄµ½Ö±ĻߵľąĄėÓėŌ²µÄ°ė¾¶Ö®²ī£¬ŅĄ“Ė¼ĘĖć¼“æÉ£»
µÄ¾ąĄėµÄ×īŠ”Öµ¼“ŹĒŌ²ŠÄµ½Ö±ĻߵľąĄėÓėŌ²µÄ°ė¾¶Ö®²ī£¬ŅĄ“Ė¼ĘĖć¼“æÉ£»
£Ø2£©¹żµć![]() ×÷
×÷![]() ÓŚ
ÓŚ![]() £¬Į¬½Ó
£¬Į¬½Ó![]() ”¢
”¢![]() £¬ĻČ¼ĘĖć³öĖıߊĪABCDµÄĆ껿ĪŖ32£¬”÷ABCµÄĆ껿ĪŖ22£¬æɵƵćPŌŚBCÉĻ£¬Ēó³ö
£¬ĻČ¼ĘĖć³öĖıߊĪABCDµÄĆ껿ĪŖ32£¬”÷ABCµÄĆ껿ĪŖ22£¬æɵƵćPŌŚBCÉĻ£¬Ēó³ö![]() £¬“Ó¶ųæɵĆAP£»
£¬“Ó¶ųæɵĆAP£»
£Ø3£©ŅŖŹ¹ĖıߊĪ![]() µÄĆ껿×īŠ”£¬Ōņ
µÄĆ껿×īŠ”£¬Ōņ![]() µÄĆ껿Šč×īŠ”£®µć
µÄĆ껿Šč×īŠ”£®µć![]() µ½
µ½![]() µÄ¾ąĄė×ī¶Ģ£¬Ōņ
µÄ¾ąĄė×ī¶Ģ£¬Ōņ![]() µÄĆ껿×īŠ”£®Ēó³ö×īŠ”
µÄĆ껿×īŠ”£®Ēó³ö×īŠ”![]() ¼“æÉ.
¼“æÉ.
£Ø1£©”ß![]() £¬
£¬![]() µÄ°ė¾¶ĪŖ
µÄ°ė¾¶ĪŖ![]() £¬
£¬
”ąŌ²ÉĻŅ»µć![]() µ½
µ½![]() µÄ¾ąĄėµÄ×īŠ”ÖµĪŖ£ŗ7-5=2£¬
µÄ¾ąĄėµÄ×īŠ”ÖµĪŖ£ŗ7-5=2£¬
¹Ź“š°øĪŖ£ŗ2£»
£Ø2£©¹żµć![]() ×÷
×÷![]() ÓŚ
ÓŚ![]() £¬Į¬½Ó
£¬Į¬½Ó![]() ”¢
”¢![]() £¬Ōņ
£¬Ōņ![]() £¬ČēĶ¼£¬
£¬ČēĶ¼£¬
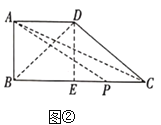
![]() £®
£®
![]() £¬
£¬
![]() µć
µć![]() ŌŚ
ŌŚ![]() ÉĻ£¬
ÉĻ£¬
![]()
![]()
![]()
£Ø3£©Į¬½Ó![]() £¬
£¬
![]() £¬µć
£¬µć![]() ĪŖ
ĪŖ![]() µÄÖŠµć£¬
µÄÖŠµć£¬
![]() £¬
£¬
![]() £¬
£¬![]() £¬
£¬
![]() ĖıߊĪ
ĖıߊĪ![]() ŹĒ¾ŲŠĪ£¬
ŹĒ¾ŲŠĪ£¬
![]() £¬
£¬![]() £¬
£¬
![]() £®
£®
![]() ŅŖŹ¹ĖıߊĪ
ŅŖŹ¹ĖıߊĪ![]() µÄĆ껿×īŠ”£¬Ōņ
µÄĆ껿×īŠ”£¬Ōņ![]() µÄĆ껿Šč×īŠ”£®
µÄĆ껿Šč×īŠ”£®
Éč![]() ĖłŌŚŌ²µÄŌ²ŠÄĪŖ
ĖłŌŚŌ²µÄŌ²ŠÄĪŖ![]() £¬Ōņ
£¬Ōņ![]() £¬¹ż
£¬¹ż![]() ×÷
×÷![]() ÓŚ
ÓŚ![]() £¬½»
£¬½»![]() ÓŚµć
ÓŚµć![]() £¬½»
£¬½»![]() ÓŚ
ÓŚ![]() £¬ÓÉ£Ø1£©æɵƓĖŹ±µć
£¬ÓÉ£Ø1£©æɵƓĖŹ±µć![]() µ½
µ½![]() µÄ¾ąĄė×ī¶Ģ£¬¼“
µÄ¾ąĄė×ī¶Ģ£¬¼“![]() µÄĆ껿×īŠ”£®
µÄĆ껿×īŠ”£®
![]() £¬
£¬
![]() £¬
£¬![]() £¬
£¬
![]() £¬
£¬![]() £¬
£¬
![]() £¬
£¬![]() £®
£®
![]() £¬
£¬
![]() £¬
£¬
![]()
![]()
![]() £¬
£¬
![]() µć
µć![]() ŌŚ
ŌŚ![]() ÉĻ£¬
ÉĻ£¬
Ōņ![]() £¬
£¬
![]() £¬
£¬
![]()
![]() £¬
£¬
![]() “ęŌŚĀś×ćÉĻŹöĢõ¼žµÄŠ”Ā·
“ęŌŚĀś×ćÉĻŹöĢõ¼žµÄŠ”Ā·![]() £¬
£¬![]() µÄ³¤ĪŖ
µÄ³¤ĪŖ![]() £®
£®


 ĆūŠ£æĪĢĆĻµĮŠ“š°ø
ĆūŠ£æĪĢĆĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗ³õÖŠŹżŃ§ Ą“Ō“£ŗ ĢāŠĶ£ŗ
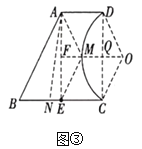
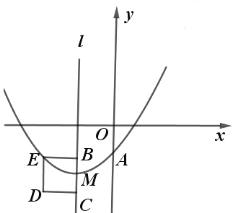
”¾ĢāÄæ”æČēĶ¼£¬ŌŚĘ½ĆęÖ±½Ē×ų±źĻµÖŠ£¬ŅŃÖŖµćA(8£¬1)£¬B(0£¬3)£¬·“±ČĄżŗÆŹż![]() (x>0)µÄĶ¼Ļó¾¹żµćA£¬¶ÆÖ±Ļßx=t(0<t<8)Óė·“±ČĄżŗÆŹżµÄĶ¼Ļó½»ÓŚµćM£¬ÓėÖ±ĻßAB½»ÓŚµćN.
(x>0)µÄĶ¼Ļó¾¹żµćA£¬¶ÆÖ±Ļßx=t(0<t<8)Óė·“±ČĄżŗÆŹżµÄĶ¼Ļó½»ÓŚµćM£¬ÓėÖ±ĻßAB½»ÓŚµćN.
(1)ĒókµÄÖµ£»
(2)Ēó”÷BMNĆ껿µÄ×ī“óÖµ£»
(3)ČōMA”ĶAB£¬ĒótµÄÖµ.
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠŹżŃ§ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æÄ³Äź¼¶¹²ÓŠ150ĆūÅ®Éś£¬ĪŖĮĖ½āøĆŠ£Å®ÉśŹµŠÄĒņ³É¼Ø£Øµ„Ī»£ŗĆ×£©ŗĶŃöĪŌĘš×ų£Øµ„Ī»£ŗøö£©µÄĒéæö£¬“ÓÖŠĖ껜³éČ”30ĆūÅ®Éś½ųŠŠ²āŹŌ£¬»ńµĆĮĖĖżĆĒµÄĻą¹Ų³É¼Ø£¬²¢¶ŌŹż¾Ż½ųŠŠÕūĄķ”¢ĆčŹöŗĶ·ÖĪö£¬ĻĀĆęøų³öĮĖ²æ·ÖŠÅĻ¢£®
![]() £®ŹµŠÄĒņ³É¼ØµÄʵŹż·Ö²¼±ķČēĻĀ£ŗ
£®ŹµŠÄĒņ³É¼ØµÄʵŹż·Ö²¼±ķČēĻĀ£ŗ
·Ö×é | 6£®2”Ü | 6£®6”Ü | 7£®0”Ü | 7£®4”Ü | 7£®8”Ü | 8£®2”Ü |
ʵŹż | 2 |
| 10 | 6 | 2 | 1 |
![]() £®ŹµŠÄĒņ³É¼ØŌŚ7£®0”Ü
£®ŹµŠÄĒņ³É¼ØŌŚ7£®0”Ü![]() £¼7£®4£®Õā×éµÄŹĒ£ŗ
£¼7£®4£®Õā×éµÄŹĒ£ŗ
7.0 | 7.0 | 7.0 | 7.1 7.1 | 7.1 | 7.2 | 7.2 | 7.3 | 7.3 |
![]() £®Ņ»·ÖÖÓŃöĪŌĘš×ų³É¼ØČēĶ¼ĖłŹ¾£ŗ
£®Ņ»·ÖÖÓŃöĪŌĘš×ų³É¼ØČēĶ¼ĖłŹ¾£ŗ

øł¾ŻŅŌÉĻŠÅĻ¢£¬»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©¢Ł±ķÖŠmµÄÖµĪŖ £»
¢Ś³éȔѧɜŅ»·ÖÖÓŃöĪŌĘš×ų³É¼ØµÄÖŠĪ»ŹżĪŖ øö£»
£Ø2£©ČōŹµŠÄĒņ³É¼Ø“ļµ½7£®2Ć×¼°ŅŌÉĻ£¬³É¼Ø¼ĒĪŖÓÅŠć£¬Ēė¹Ą¼ĘČ«Äź¼¶Å®Éś³É¼Ø“ļµ½ÓÅŠćµÄČĖŹż£®
£Ø3£©øĆÄź¼¶Ä³°ąĢåÓżĪÆŌ±½«±¾°ąŌŚÕā“Ī³éŃł²āŹŌÖŠ±»³éČ”µÄ8ĆūÅ®ÉśµÄĮ½Ļī³É¼ØµÄŹż¾Ż³Ā¼ČēĻĀ£ŗ
Å®Éś“śĀė | A | B | C | D | E | F | G | H |
ŹµŠÄĒņ | 8£®1 | 7£®7 | 7£®5 | 7£®5 | 7£®3 | 7£®2 | 7£®0 | 6£®5 |
Ņ»·ÖÖÓŃöĪŌĘš×ų | * | 42 | 47 | * | 47 | 52 | * | 49 |
ĘäÖŠÓŠ2ĆūÅ®ÉśµÄŅ»·ÖÖÓŃöĪŌĘš×ų³É¼ØĪ“³Ā¼ĶźÕū£¬µ±ĄĻŹ¦ĖµÕā8ĆūÅ®ÉśĒ”ŗĆÓŠ4ČĖĮ½Ļī²āŹŌ³É¼Ø¶¼“ļµ½ĮĖÓÅŠć£¬ÓŚŹĒĢåÓżĪÆŌ±ĶĘ²āÅ®ÉśEµÄŅ»·ÖÖÓŃöĪŌĘš×ų³É¼Ø“ļµ½ĮĖÓÅŠć£¬ÄćĶ¬ŅāĢåÓżĪÆŌ±µÄĖµ·ØĀš£æ²¢ĖµĆ÷ÄćµÄĄķÓÉ£®
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠŹżŃ§ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æŌŚĘ½ĆęÖ±½Ē×ų±źĻµÖŠ£¬µć![]() £¬µć
£¬µć![]() £®½«
£®½«![]() Čʵć
Čʵć![]() Ė³Ź±ÕėŠż×Ŗ£¬µĆ
Ė³Ź±ÕėŠż×Ŗ£¬µĆ![]() £¬µć
£¬µć![]() £¬
£¬![]() Šż×ŖŗóµÄ¶ŌÓ¦µćĪŖ
Šż×ŖŗóµÄ¶ŌÓ¦µćĪŖ![]() £¬
£¬![]() £®¼ĒŠż×Ŗ½ĒĪŖ
£®¼ĒŠż×Ŗ½ĒĪŖ![]() £®
£®
£Ø1£©ČēĶ¼¢Ł£¬µ±![]() Ź±£¬Ēóµć
Ź±£¬Ēóµć![]() µÄ×ų±ź£»
µÄ×ų±ź£»
£Ø2£©ČēĶ¼¢Ś£¬µ±![]() Ź±£¬Ēóµć
Ź±£¬Ēóµć![]() µÄ×ų±ź£»
µÄ×ų±ź£»
£Ø3£©Į¬½Ó![]() £¬ÉčĻ߶Ī
£¬ÉčĻ߶Ī![]() µÄÖŠµćĪŖ
µÄÖŠµćĪŖ![]() £¬Į¬½Ó
£¬Į¬½Ó![]() £¬ĒóĻ߶Ī
£¬ĒóĻ߶Ī![]() µÄ³¤µÄ×īŠ”Öµ£ØÖ±½ÓŠ“³ö½į¹ū¼“æÉ£©£®
µÄ³¤µÄ×īŠ”Öµ£ØÖ±½ÓŠ“³ö½į¹ū¼“æÉ£©£®
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠŹżŃ§ Ą“Ō“£ŗ ĢāŠĶ£ŗ
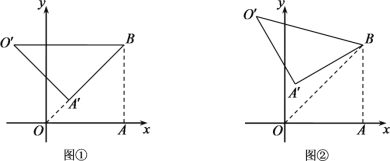
”¾ĢāÄæ”æČēĶ¼£¬ŌŚ![]() ÖŠ£¬
ÖŠ£¬![]() £¬
£¬![]() £¬ŅŌ
£¬ŅŌ![]() ĪŖ±ßŌŚ
ĪŖ±ßŌŚ![]() Ķā×÷Õż·½ŠĪ
Ķā×÷Õż·½ŠĪ![]() £¬
£¬![]() ”¢
”¢![]() ½»ÓŚµć
½»ÓŚµć![]() £¬ŌņĻ߶Ī
£¬ŌņĻ߶Ī![]() µÄ×ī“óÖµĪŖ_______£®
µÄ×ī“óÖµĪŖ_______£®
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠŹżŃ§ Ą“Ō“£ŗ ĢāŠĶ£ŗ
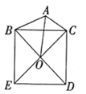
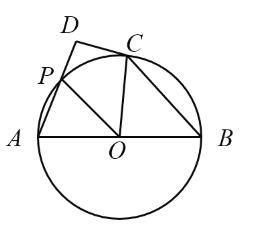
”¾ĢāÄæ”æ¶ØŅå£ŗČēĶ¼1£¬ŅŃÖŖČń½Ē![]() ÄŚÓŠ¶Øµć
ÄŚÓŠ¶Øµć![]() £¬¹żµć
£¬¹żµć![]() ČĪŅā×÷Ņ»ĢõÖ±Ļß
ČĪŅā×÷Ņ»ĢõÖ±Ļß![]() £¬·Ö±š½»ÉäĻß
£¬·Ö±š½»ÉäĻß![]() £¬
£¬![]() ÓŚµćM£¬N£®Čō
ÓŚµćM£¬N£®Čō![]() ŹĒĻ߶Ī
ŹĒĻ߶Ī![]() µÄÖŠµćŹ±£¬Ōņ³ĘÖ±Ļß
µÄÖŠµćŹ±£¬Ōņ³ĘÖ±Ļß![]() ŹĒ
ŹĒ![]() µÄÖŠµćÖ±Ļߣ®ČēĶ¼2£¬ÉäĻß
µÄÖŠµćÖ±Ļߣ®ČēĶ¼2£¬ÉäĻß![]() µÄ½āĪöŹ½ĪŖ
µÄ½āĪöŹ½ĪŖ![]() Óė
Óė![]() ÖįµÄ¼Š½ĒĪŖ
ÖįµÄ¼Š½ĒĪŖ![]() £¬
£¬![]() £¬
£¬![]() ĪŖ
ĪŖ![]() µÄÖŠµćÖ±Ļߣ®
µÄÖŠµćÖ±Ļߣ®
£Ø1£©ĒóÖ±Ļß![]() µÄ½āĪöŹ½£»
µÄ½āĪöŹ½£»
£Ø2£©Čō¹żµć![]() ČĪŅā×÷Ņ»ĢõÖ±Ļß
ČĪŅā×÷Ņ»ĢõÖ±Ļß![]() £¬·Ö±š½»ÉäĻß
£¬·Ö±š½»ÉäĻß![]() £¬
£¬![]() ÖįµÄÕż°ėÖįÓŚµć
ÖįµÄÕż°ėÖįÓŚµć![]() £¬
£¬![]() £¬¼Ē
£¬¼Ē![]() µÄĆ껿ĪŖ
µÄĆ껿ĪŖ![]() £¬
£¬![]() µÄĆ껿ĪŖ
µÄĆ껿ĪŖ![]() £®ĒóÖ¤£ŗ
£®ĒóÖ¤£ŗ![]() £®
£®
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠŹżŃ§ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æČēĶ¼£¬Å×ĪļĻß![]()
![]() ¹żµć
¹żµć![]() £¬¶„µć
£¬¶„µć![]() ŌŚµŚČżĻóĻŽ£¬
ŌŚµŚČżĻóĻŽ£¬![]() £¬
£¬![]() ŹĒÅ×ĪļĻߵĶŌ³ĘÖį
ŹĒÅ×ĪļĻߵĶŌ³ĘÖį![]() ÉĻµÄĮ½µć£¬ĒŅ
ÉĻµÄĮ½µć£¬ĒŅ![]() £¬ŌŚÖ±Ļß
£¬ŌŚÖ±Ļß![]() ×ó²ąŅŌ
×ó²ąŅŌ![]() ĪŖ±ß×÷Õż·½ŠĪ
ĪŖ±ß×÷Õż·½ŠĪ![]() £¬µć
£¬µć![]() Ē”ŗĆŌŚÅ×ĪļĻßÉĻ£®
Ē”ŗĆŌŚÅ×ĪļĻßÉĻ£®
£Ø1£©ÓĆŗ¬![]() µÄŹ½×Ó±ķŹ¾
µÄŹ½×Ó±ķŹ¾![]() £»
£»
£Ø2£©ĒóÖ¤£ŗµć![]() ŗĶµć
ŗĶµć![]() ¹ŲÓŚÖ±Ļß
¹ŲÓŚÖ±Ļß![]() ¶Ō³Ę£»
¶Ō³Ę£»
£Ø3£©ÅŠ¶ĻÖ±Ļß![]() ŗĶÖ±Ļß
ŗĶÖ±Ļß![]() £Ø
£Ø![]() ŹĒ³£Źż£¬ĒŅ
ŹĒ³£Źż£¬ĒŅ![]() £©µÄ½»µćŹĒ·ńŌŚÅ×ĪļĻßÉĻ£¬²¢ĖµĆ÷ĄķÓÉ£®
£©µÄ½»µćŹĒ·ńŌŚÅ×ĪļĻßÉĻ£¬²¢ĖµĆ÷ĄķÓÉ£®
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠŹżŃ§ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æČēĶ¼£¬ŅŃÖŖ![]() ŹĒ
ŹĒ![]() µÄÖ±¾¶£¬µć
µÄÖ±¾¶£¬µć![]() ŹĒ
ŹĒ![]() ÉĻŅ»µć£¬Į¬½Ó
ÉĻŅ»µć£¬Į¬½Ó![]() £¬µć
£¬µć![]() ¹ŲÓŚ
¹ŲÓŚ![]() µÄ¶Ō³Ęµć
µÄ¶Ō³Ęµć![]() Ē”ŗĆĀäŌŚ
Ē”ŗĆĀäŌŚ![]() ÉĻ£®
ÉĻ£®
£Ø1£©ĒóÖ¤£ŗ![]() £»
£»
£Ø2£©¹żµć![]() ×÷
×÷![]() µÄĒŠĻß
µÄĒŠĻß![]() £¬½»
£¬½»![]() µÄŃÓ³¤ĻßÓŚµć
µÄŃÓ³¤ĻßÓŚµć![]() £®Čē¹ū
£®Čē¹ū![]() £¬Ēó
£¬Ēó![]() µÄÖ±¾¶£®
µÄÖ±¾¶£®
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠŹżŃ§ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æÄ³ÖŠŃ§°ĖÄź¼¶Ń§ÉśŌŚŗ®¼ŁĘŚ¼ä»ż¼«æ¹»÷ŅßĒ飬æŖÕ¹ĄĻŹ¦”°ŌŚÄćÉķ±ß”±ĘĄŠĒ»ī¶Æ£¬Ń§ÉśæÉŅŌ“Ó”°×ŌĄķŠĒ”± ”¢”°¶ĮŹéŠĒ”±”¢”°½”浊Ē”±”¢”°Š¢¾“ŠĒ”±”¢”° ĄĶ¶ÆŠĒ”±µČÖŠŃ”Ņ»øöĻīÄæ²Ī¼ÓÕłŠĒ¾ŗŃ”£¬øł¾ŻøĆŠ£°ĖÄź¼¶Ń§ÉśµÄ”°ÕłŠĒ”±±ØĆūĒéæö£¬»ęÖĘ³ÉĮĖČēĻĀĮ½·ł²»ĶźÕūµÄĶ³¼ĘĶ¼£¬Ēėøł¾ŻĶ¼ÖŠŠÅĻ¢»Ų“šĻĀĮŠĪŹĢā£ŗ
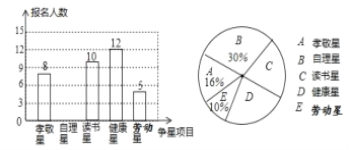
£Ø1£©²Ī¼ÓÄź¼¶ĘĄŠĒµÄѧɜ¹²ÓŠ________ČĖ£»½«ĢõŠĪĶ³¼ĘĶ¼²¹³äĶźÕū£»
£Ø2£©ÉČŠĪĶ³¼ĘĶ¼ÖŠ”°¶ĮŹéŠĒ”±¶ŌÓ¦µÄÉČŠĪŌ²ŠÄ½Ē¶ČŹżŹĒ________£»
£Ø3£©Čō°ĖÄź¼¶1°ą×¼±øĶĘ¼ö¼×”¢ŅŅ”¢±ū”¢¶”ĖÄĆūĶ¬Ń§ÖŠµÄ2Ćū“ś±ķ°ą¼¶²Ī¼ÓѧŠ£µÄ”°ĄĶ¶ÆŠĒ”± ±ØĆū£¬ĒėÓƱķøń»ņŹ÷דĶ¼·ÖĪö¼×ŗĶŅŅĶ¬Ń§Ķ¬Ź±±»Ń”ÖŠµÄøÅĀŹ£®
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com