(13��)���A��C
10H
20O
2������������ζ����֪��
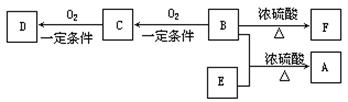
��B������û��֧����
��D����̼��������Һ��Ӧ�ų�������̼��
��D��E��Ϊ������ͬ�����ŵ�ͬ���칹�塣E���������ϵ�������Clȡ������һ�ȴ���ֻ��һ�֡���F����ʹ������Ȼ�̼��Һ��ɫ��
��1��B���Է����ķ�Ӧ��
�� ��ѡ����ţ���
��ȡ����Ӧ ����ȥ��Ӧ �ۼӾ۷�Ӧ ��������Ӧ
��2��D��F���������Ĺ����ŵ�����������
�� ��
�� ��
��3��д����D��E������ͬ����������ͬ���칹��Ľṹ��ʽ��
�� ��
��4��B��E��Ӧ����A�Ļ�ѧ��Ӧ����ʽ
�� ��5��ijѧ������C�Ĺ�����ʱ��ȡ1mol/LCuSO
4��Һ��2mol/LNaOH��Һ��1mL����һ֧�ྻ���Թ��ڻ�Ϻ��������ּ���0.5mL40%��C�����Ⱥ���ɫ�������֡���ͬѧʵ��ʧ�ܵ�ԭ�������
�� ��ѡ����ţ�
�ټ����C���� �ڼ����C̫��
�ۼ���CuSO
4��Һ�������� �ܼ���CuSO
4��Һ��������


