
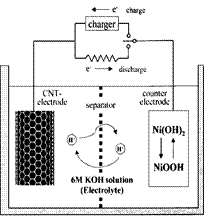
| A£®Õō·¢Ćó | B£®ŹÆĆŽĶų | C£®ÄąČż½Ē | D£®ÉÕ± E£®ŪįŪöĒÆ F£®¾Ę¾«µĘ |
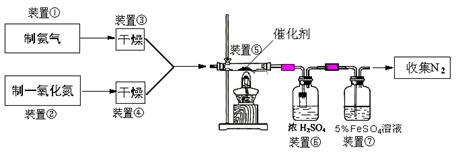
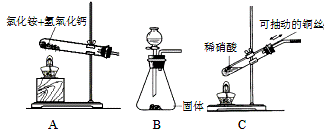
 Cr2O3”¤2CuO+N2”ü+5H2O£Ø²śĪļŠ““ķ”¢²»ÅäĘ½²»øų·Ö£©”£
Cr2O3”¤2CuO+N2”ü+5H2O£Ø²śĪļŠ““ķ”¢²»ÅäĘ½²»øų·Ö£©”£ CaCl2+2NH3”ü +2H2O£»ÅØ°±Ė®”¢¼īŹÆ»Ņ(ÉśŹÆ»Ņ»ņĒāŃõ»ÆÄĘ¹ĢĢå)
CaCl2+2NH3”ü +2H2O£»ÅØ°±Ė®”¢¼īŹÆ»Ņ(ÉśŹÆ»Ņ»ņĒāŃõ»ÆÄĘ¹ĢĢå)


| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗ³õÖŠŹżŃ§ Ą“Ō“£ŗ ĢāŠĶ£ŗ

²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠŹżŃ§ Ą“Ō“£ŗ ĢāŠĶ£ŗ
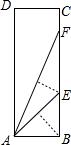 ½«ČēĶ¼µÄ¾ŲŠĪÖ½Ę¬ABCDŃŲ¹żµćBµÄÖ±ĻßÕŪµž£¬Ź¹µćAĀäŌŚBCÉĻµÄµćE“¦£¬»¹Ōŗó£¬ŌŁŃŲ¹żµćEµÄÖ±ĻßÕŪµž£¬Ź¹µćAĀäŌŚBCÉĻµÄµćF“¦£¬ÕāŃł¾ĶæÉŅŌĒó³ö”ĻAFEµÄÕżĒŠÖµŹĒ£Ø””””£©
½«ČēĶ¼µÄ¾ŲŠĪÖ½Ę¬ABCDŃŲ¹żµćBµÄÖ±ĻßÕŪµž£¬Ź¹µćAĀäŌŚBCÉĻµÄµćE“¦£¬»¹Ōŗó£¬ŌŁŃŲ¹żµćEµÄÖ±ĻßÕŪµž£¬Ź¹µćAĀäŌŚBCÉĻµÄµćF“¦£¬ÕāŃł¾ĶæÉŅŌĒó³ö”ĻAFEµÄÕżĒŠÖµŹĒ£Ø””””£©²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠŹżŃ§ Ą“Ō“£ŗ ĢāŠĶ£ŗ
 Š”Ć÷ŌŚŃ§Ļ°”°Čń½ĒČż½ĒŗÆŹż”±ÖŠ·¢ĻÖ£¬½«ČēĶ¼ĖłŹ¾µÄ¾ŲŠĪÖ½Ę¬ABCDŃŲ¹ż µćBµÄÖ±ĻßÕŪµž£¬Ź¹µćAĀäŌŚBCÉĻµÄµćE“¦£¬»¹Ōŗó£¬ŌŁŃŲ¹żµćEµÄÖ±ĻßÕŪµž£¬Ź¹µćAĀäŌŚBCÉĻµÄµćF“¦£¬ÕāŃł¾ĶæÉŅŌĒó³ö
Š”Ć÷ŌŚŃ§Ļ°”°Čń½ĒČż½ĒŗÆŹż”±ÖŠ·¢ĻÖ£¬½«ČēĶ¼ĖłŹ¾µÄ¾ŲŠĪÖ½Ę¬ABCDŃŲ¹ż µćBµÄÖ±ĻßÕŪµž£¬Ź¹µćAĀäŌŚBCÉĻµÄµćE“¦£¬»¹Ōŗó£¬ŌŁŃŲ¹żµćEµÄÖ±ĻßÕŪµž£¬Ź¹µćAĀäŌŚBCÉĻµÄµćF“¦£¬ÕāŃł¾ĶæÉŅŌĒó³ö| 2 |
| 2 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠŹżŃ§ Ą“Ō“£ŗ ĢāŠĶ£ŗ
 ČēĶ¼£¬¾ŲŠĪÖ½Ę¬ABCDŃŲ¹żµćBµÄÖ±ĻßÕŪµž£¬µćAĀäŌŚBC±ßÉĻµÄµćE“¦£¬»¹Ōŗó£¬ŌŁŃŲ¹żµćEµÄÖ±ĻßÕŪµž£¬µćAĀäŌŚBC±ßÉĻµÄµćF“¦£¬Ōņtan”ĻFAB=£Ø””””£©
ČēĶ¼£¬¾ŲŠĪÖ½Ę¬ABCDŃŲ¹żµćBµÄÖ±ĻßÕŪµž£¬µćAĀäŌŚBC±ßÉĻµÄµćE“¦£¬»¹Ōŗó£¬ŌŁŃŲ¹żµćEµÄÖ±ĻßÕŪµž£¬µćAĀäŌŚBC±ßÉĻµÄµćF“¦£¬Ōņtan”ĻFAB=£Ø””””£©²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com