
【题目】听下面一段对话,完成五道小题,每小题仅填写一个词。这段对话你将听两遍。
【答案】
(1) military school
(2) French army
(3) general
(4) Emperor of
(5) defeat/end
【解析】听力原文:
Napoleon was a French soldier who became Emperor of France. He was born in 1769 on the island of Corsica. When he was only ten years old, his father sent him to a military school in France. Napoleon was not a very good student in most of his classes, but he excelled in mathematics and military science. When he was sixteen years old, he joined the French army. In that year he began the military career that brought him fame, power, wealth, and finally, defeat. Napoleon became a general in the French army at the young age of twenty-four. Several years later he became Emperor of the French Empire.
Napoleon was many things. He was, first of all, a good military leader. His soldiers were ready to die for him. As a result, Napoleon won many, many military victories. At one time he controlled most of Europe, but many countries, including England, Russia, and Austria fought against Napoleon. His defeat--his end--came when he decided to attack Russia. In this military campaign into Russia, he lost most of his army.
The great French conqueror died alone--deserted by his family and his friends--in 1821. Napoleon was only fifty-one years old when he died.


科目:高中地理 来源: 题型:
【题目】(6分)(1)如图所示为研究平行板电容器电容的实验。电容器充电后与电源断开,电荷量Q 将不变,与电容器相连的静电计用来测量电容器的____________。在常见的电介质中,由于空气的介电常数是最小的,当极板间插入其他的电介质板时,电容器的电容将_________(选填“增大”“减小”或“不变”),于是我们发现,静电计指针偏角将__________。(选填“增大”“减小”或“不变”)
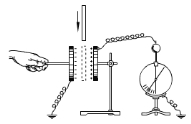
(2)连接在电源两极板上的平行板电容器,当两极板间的距离减小时,电容器的电容C将__________,带电荷量Q将______,极板间的电场强度E将________。(选填“增大”“减小”或“不变”)
查看答案和解析>>
科目:高中地理 来源: 题型:
【题目】(6分)用滴水法可以测定重力加速度的值,方法是:在自来水龙头下面固定一挡板,如图所示,仔细调节水龙头,使得前一个水滴滴在挡板上的同时,下一个水滴刚好开始下落.首先量出水龙头口离挡板的高度h,再用秒表计时,计时的方法是:当听到某一水滴滴在挡板上的声音的同时,开启秒表开始计时,并数1,以后每听到一滴水声,依次数2、3、4……一直数到n时,按下秒表按钮停止计时,读出秒表的读数t。
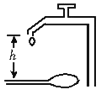
(1)写出用上述方法测量重力加速度g的表达式g=____________。
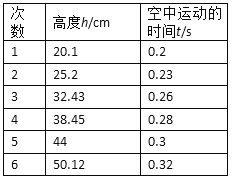
(2)为了减小误差,改变h的数值,测出多组数据,记录在表格中。表格中的t是水滴从水龙头口到挡板所用的时间,即水滴在空中运动的时间,请在坐标纸中作出适当的图象,并利用图象求出重力加速度的值g=______m/s2。(保留两位有效数字)
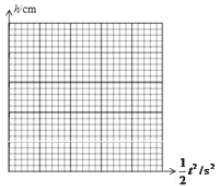
查看答案和解析>>
科目:高中地理 来源: 题型:
【题目】有机物A只由C、H、O三种元素组成,常用作有机合成的中间体,测得16.8 g该有机物完全燃烧生成44.0 g CO2和14.4 g水。质谱图表明其相对分子质量为84;红外光谱分析表明A中含有—O—H和位于分子端的C![]() CH,核磁共振氢谱显示有3种峰,且峰面积之比为6∶1∶1。
CH,核磁共振氢谱显示有3种峰,且峰面积之比为6∶1∶1。
(1)写出A的分子式____________;
(2)写出A的结构简式____________;
(3)下列物质一定能与A发生反应的是____________(填序号)
a.H2 b.Na
c.KMnO4 d.Br2
(4)有机物B是A的同分异构体,1 mol B可以与1 mol Br2加成,该有机物的所有碳原子在同一平面上,且没有顺反异构现象,则B的结构简式是______________________。
查看答案和解析>>
科目:高中地理 来源: 题型:
【题目】将1 mol CH4和适量O2在密闭容器中混合点燃,充分反应后,CH4和O2均无剩余,且产物均为气体,质量为72 g,下列叙述正确的是( )
A.若将产物通过碱石灰,则可全部被吸收;若通入浓硫酸,则不能完全被吸收
B.产物的平均摩尔质量为20 g·mol-1
C.若将产物通过浓硫酸后恢复至室温,压强变为反应前的![]()
D.反应中消耗O256 g
查看答案和解析>>
科目:高中地理 来源: 题型:
【题目】如图是某同学设计的制取氯气以及验证氯气的性质的实验,回答下列问题
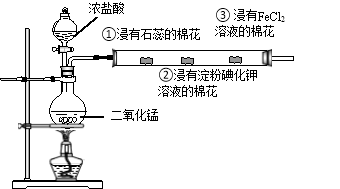
(1)该同学制取氯气的反应原理是: (写化学方程式)。
(2)实验过程中②处观察到的现象是 说明氯气具有的性质是 。
(3)该实验操作的不足之处是
(4)实验室用MnO2和浓盐酸制取Cl2时,有14.6 g氯化氢被氧化,所得Cl2全部用NaOH溶液吸收,生成NaClO的物质的量是 moi
查看答案和解析>>
科目:高中地理 来源: 题型:
【题目】A、B、C、D、E、G、H、I、J是中学化学常见的9种化合物,F、H常温下呈气态,B常温下呈液态,其中F是人类生存不能离开的气体单质,反应③常用于焊接和切割金属,其转化关系如下图,据此回答下列问题:
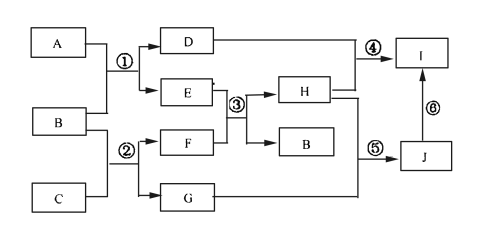
(1)写出C的电子式__________________。
(2)写出反应①的化学方程式______________________。
(3)列出两种E能发生的反应类型_________________________。
(4)写出过量的H与D溶液反应的离子方程式________________。
(5)实验室制取H的装置可以选用下列装置中的(填下图中的选项字母)。
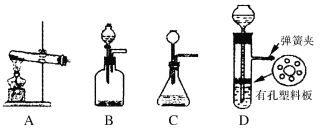
查看答案和解析>>
湖北省互联网违法和不良信息举报平台 | 网上有害信息举报专区 | 电信诈骗举报专区 | 涉历史虚无主义有害信息举报专区 | 涉企侵权举报专区
违法和不良信息举报电话:027-86699610 举报邮箱:58377363@163.com