
����Ŀ��[2017�¿α���]Li4Ti5O12��LiFePO4��������ӵ�صĵ缫���ϣ���������������Ҫ�ɷ�ΪFeTiO3������������MgO��SiO2�����ʣ����Ʊ��������������£�
�ش��������⣺
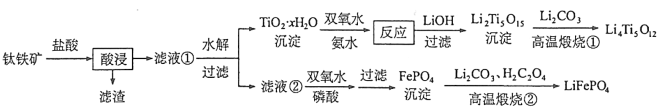
��1���������ʵ���У����Ľ����ʽ������ͼ��ʾ����ͼ��֪�������Ľ�����Ϊ70%ʱ�������õ�ʵ������Ϊ___________________��
��2���������������Ҫ��![]() ��ʽ���ڣ�д����Ӧ��Ӧ�����ӷ���ʽ__________________��
��ʽ���ڣ�д����Ӧ��Ӧ�����ӷ���ʽ__________________��
��3��TiO2��xH2O������˫��ˮ����ˮ��Ӧ40 min����ʵ�������±���ʾ��
�¶�/�� | 30 | 35 | 40 | 45 | 50 |
TiO2��xH2Oת����% | 92 | 95 | 97 | 93 | 88 |
����40 ��ʱTiO2��xH2Oת������ߵ�ԭ��__________________��
��4��Li2Ti5O15��Ti�Ļ��ϼ�Ϊ+4�����й���������ĿΪ__________________��
��5��������Һ������![]() ������˫��ˮ�����ᣨ����Һ�������1������ʹ
������˫��ˮ�����ᣨ����Һ�������1������ʹ![]() ǡ�ó�����ȫ����Һ��
ǡ�ó�����ȫ����Һ��![]() ����ʱ�Ƿ���Mg3(PO4)2�������ɣ� ����ʽ���㣩��FePO4��Mg3(PO4)2��Ksp�ֱ�Ϊ
����ʱ�Ƿ���Mg3(PO4)2�������ɣ� ����ʽ���㣩��FePO4��Mg3(PO4)2��Ksp�ֱ�Ϊ![]() ��
��
��6��д��������������������FePO4�Ʊ�LiFePO4�Ļ�ѧ����ʽ ��
���𰸡���1��100����2h��90����5h
��2��FeTiO3+ 4H++4Cl = Fe2++ ![]() + 2H2O
+ 2H2O
��3������40����TiO2��xH2Oת����Ӧ�������¶����߶����ӣ�����40����˫��ˮ�ֽ��백���ݳ�����TiO2��xH2Oת����Ӧ�����½�
��4��4
��5��Fe3+ǡ�ó�����ȫʱ��c(![]() )=
)=![]() mol��L1=1.3��10�C17 mol��L1��c3(Mg2+)��c2(
mol��L1=1.3��10�C17 mol��L1��c3(Mg2+)��c2(![]() )��(0.01)3��(1.3��10�C17)2=1.7��10�C40��Ksp [Mg3(PO4)2]����˲�������Mg3(PO4)2������
)��(0.01)3��(1.3��10�C17)2=1.7��10�C40��Ksp [Mg3(PO4)2]����˲�������Mg3(PO4)2������
��6��2FePO4 + Li2CO3+ H2C2O4![]() 2LiFePO4+ H2O��+ 3CO2��
2LiFePO4+ H2O��+ 3CO2��
����������1����ͼʾ��֪���������ʱ���ľ�����Ϊ70%ʱ����Ӧѡ����100����2h��90����5h�½��У�
��2���������ʱ�������ܽ�FeTiO3����![]() ʱ��������Ӧ�����ӷ���ʽΪFeTiO3+4H++4Cl��Fe2++
ʱ��������Ӧ�����ӷ���ʽΪFeTiO3+4H++4Cl��Fe2++ ![]() + 2H2O��
+ 2H2O��
��3���¶���Ӱ�����ʵ���Ҫ���أ���H2O2�ڸ������ֽ⡢��ˮ�ӷ�����ԭ���ǵ���40����TiO2��xH2Oת����Ӧ�������¶����߶����ӣ�����40����˫��ˮ�ֽ��백���ݳ�����TiO2��xH2Oת����Ӧ�����½���
��4��Li2Ti5O15��LiΪ+1�ۣ�OΪ�C2�ۣ�TiΪ+4�ۣ�������(![]() )����Ԫ���ԨC1�ۣ������������ĿΪx�������������ϼ۴�����Ϊ0����֪(+1)��2+(+4)��5+(�C2)��(15�C2x)+(�C1)��2x=0����ã�x=4��
)����Ԫ���ԨC1�ۣ������������ĿΪx�������������ϼ۴�����Ϊ0����֪(+1)��2+(+4)��5+(�C2)��(15�C2x)+(�C1)��2x=0����ã�x=4��
��5��Ksp[FePO4]=c(Fe3+)��c(![]() )=1.3��10�C2����c(
)=1.3��10�C2����c(![]() )��
)��![]() ��1.3��10�C17 mol/L��Qc[Mg3(PO4)2]��c3(Mg2+)��c2(
��1.3��10�C17 mol/L��Qc[Mg3(PO4)2]��c3(Mg2+)��c2(![]() )��(0.01)3��(1.3��10�C17)2=1.69��10�C40��1.0��10�C24����������
)��(0.01)3��(1.3��10�C17)2=1.69��10�C40��1.0��10�C24����������
��6��������FePO4��Li2CO3��H2C2O4��ϼ��ȿɵ�LiFePO4�����ݵ����غ��ԭ���غ�ɵô˷�Ӧ�Ļ�ѧ����ʽΪ2FePO4 + Li2CO3+ H2C2O4![]() 2LiFePO4+ H2O��+ 3CO2����
2LiFePO4+ H2O��+ 3CO2����



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����е��� ��Դ�� ���ͣ�
����Ŀ����������Ũ�Ⱦ�Ϊ0.5 mol/L��������Һ��
��Na2CO3����NaHCO3����HCl����NH3��H2O
��1��������Һ�У��ɷ���ˮ�����_________(����ţ���ͬ)��
��2��������Һ�У��������������Ʒ�Ӧ�����ܺ����ᷴӦ����Һ������Ũ���ɴ�С��˳��Ϊ��_____________________________________��
��3�������м��������Ȼ�粒��壬��ʱc(![]() )/c(OH��)��ֵ________(�������С�����䡱)��
)/c(OH��)��ֵ________(�������С�����䡱)��
��4����������������Һ��Ϻ���Һǡ�ó����ԣ�����ǰ�������________�������(����ڡ�����С�ڡ����ڡ�)����ʱ��Һ������Ũ���ɴ�С��˳����____________________________________��
��5��ȡ10 mL��Һ������ˮϡ�͵�500 mL�����ʱ��Һ����ˮ�������c(H+)=_______________��
�鿴�𰸺ͽ���>>
��Ŀ�����е��� ��Դ�� ���ͣ�
����Ŀ����������ˮ�ķ�Ӧ����ѧ��ѧ�е�һ����Ҫ��Ӧ���̲��и�ʵ�������ֹ����ֲ�ͬ����ʾ�������ֱ�����ͼ�мס��ҡ�����ʾ��
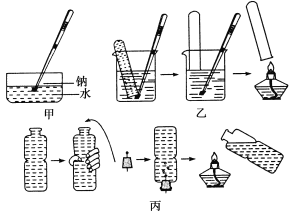
(1)�ְ�ͼ����ʾ�ķ�����������ʱ����ʢ�б���Ca(OH)2��Һ��ˮ���У�����һС������ơ���������������ȷ����________(����ĸ)��
A���Ƹ���Һ���ϣ����Ĵ��ζ��������ʧ
B�����ۻ���һ��������С��
C����Һ�ײ�������ɫ��������
D���ָ�������ʱ��ˮ�۵ײ��й�����������
(2)�벹�䲢��ɼ�ʵ���д��Լ�ƿ��ȡ���Ƶ���ˮ����Ͷ���Ƶ��йز����������Ӵ��Լ�ƿ��ȡ��һС���ơ�__________________�������Ӽ�ȡ�кõĽ�����Ͷ�뵽ʢ��Ca(OH)2��Һ��ˮ���С�
(3)ijͬѧ�����Ӽ�סһ���ƣ���ͼ����ʾ�������ռ����������壬���ƺܿ����䣬ʵ��û�гɹ�����ȷ�IJ���ӦΪ________________________________________________��
(4)��װ��ˮ�Ŀ�Ȫˮƿ��ͼ����������ʵ�顣���ּ�ѹ��Ȫˮƿ�����������Ƶ����ӡ����ų�ˮ�����Ϊ16.8 mL�����Ʒ�Ӧ�����ɱ�Ĵ�����Ȫˮƿ�ָ���ԭ״������ƿ�ڡ�ƿ������ڱ�״���������õ��Ƶ�����Ϊ________��
�鿴�𰸺ͽ���>>
��Ŀ�����е��� ��Դ�� ���ͣ�
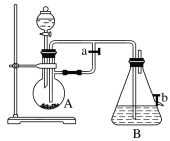
����Ŀ����ͼ��ʾװ�ã���������ȡ�۲�Fe(OH)2�ڿ����б����������е���ɫ�仯��ʵ��ʱ����ʹ����м��
6 mol��L1�����ᣬ�����Լ���ѡ��
��ش��������⣺
(1)B��ʢ��һ������NaOH��Һ��A��ӦԤ�ȼ����������__________________��A�з�Ӧ�����ӷ���ʽ��____________________________________________________��
(2)ʵ�鿪ʼʱӦ�Ƚ�����a________(����رա�)����Ŀ����____________________��
(3)��������Fe(OH)2�IJ������̣�__________________________________________��
(4)ʵ����ϣ���b������������һ���ֿ�������ʱB�з�����Ӧ�Ļ�ѧ����ʽΪ_________________��
(5)ͼ��________(��ܡ����ܡ�)�ϳ�ʱ�俴��Fe(OH)2��ɫ������
�鿴�𰸺ͽ���>>
��Ŀ�����е��� ��Դ�� ���ͣ�
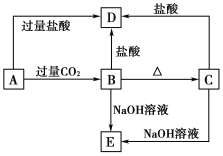
����Ŀ��A��B��C��D��E���ֻ����������ij�ֳ���Ԫ�أ����ǵ�ת����ϵ����ͼ��ʾ������AΪ������Һ��CΪ������ˮ�İ�ɫ���壬E��������ˮ����ȡA��Һ���գ���ɫ��ӦΪdz��ɫ(����ɫ�ܲ���)��
��ش��������⣺
(1)д����ѧʽ��A__________��B__________��C__________��D______��E________��
(2)д�����з�Ӧ�����ӷ���ʽ��
A��B��________________________________��
B��D��________________________________��
C��E��________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����е��� ��Դ�� ���ͣ�
����Ŀ��ij����С��ģ���������е�ԭ��(���������볱ʪ������̼��Ӧ)�������������������ȡ���������������������
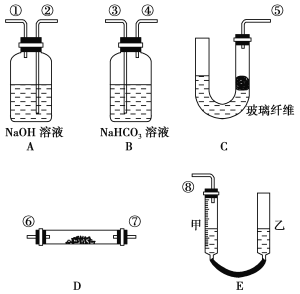
��ͼ������װ��E���ɼס���������������ɣ���������Ƥ����ͨ����װ������ˮ�����п̶�(0��50 mL)���������ã��ҹܿ������ƶ����Ե���Һ��ߵ͡�
ʵ���ҿɹ�ѡ�õ�ҩƷ���У�ϡ���ᡢ���ᡢ�������ơ�̼���ơ�����ʯ��ˮ������װ�õ�����˳�����ݢۢܢޢߢڢ٢�
(��ʾ��Bװ��������ȥ�Ƶõ�CO2�е�HCl)
�Իش�
(1)д��װ��D�еĻ�ѧ��Ӧ����ʽ ________________________________________________��
(2)Cװ��������ȡCO2��д����װ���еĻ�ѧ��Ӧ����ʽ��_______________________________��
(3)װ��A��������__________________________________________________________��
(4)Ϊ�˽�ȷ�ز�����������������˱���������װ�õ�������֮�⣬�ڶ�ȡ��Ӧǰ�����Һ��Ķ����������ֵ�Ĺ����У�Ӧע��________(��д��ĸ���)��
a�������밼Һ����ʹ���ƽ
b���ȴ�Ƭ�̣����ҹ���Һ�治������ʱ�����̶���
c������ʱӦ�����ƶ��ҹܣ�ʹ�ס�������Һ����ƽ
d������ʱ��һ��ʹ�ס�������Һ����ƽ
�鿴�𰸺ͽ���>>
��Ŀ�����е��� ��Դ�� ���ͣ�
����Ŀ��Choose one skill and use specific reasons and examples, with nothing _______ going on, to support your choice.
A. typical B. particular C. abstract D. convincing
�鿴�𰸺ͽ���>>
��Ŀ�����е��� ��Դ�� ���ͣ�
����Ŀ��(2014���㽭) Facing up to your problem _________ running away from them is the best approach to working things out.
A. more than B. rather than
C. along with D. or rather
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com