
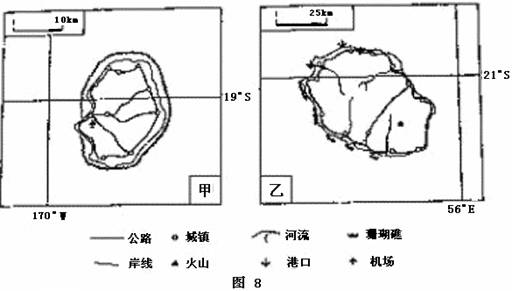
Ķ¼8ĪŖ¼×”¢ŅŅĮ½µŗĀŌĶ¼£¬ĘäÖŠ¼×µŗµŲŹĘµĶĘ½”£Ķź³ÉĻĀĮŠŅŖĒó”£

£Ø1£©°“¶«”¢Ī÷°ėĒņ»®·Ö£¬¼×µŗĪ»ÓŚ£Ø £©°ėĒņ”£¼×µŗÖÜĪ§µÄĖ®ÓņŹōÓŚ£Ø £©Ńó£¬ŅŅµŗÖÜĪ§µÄĖ®ÓņŹōÓŚ£Ø £©Ńó”£
£Ø2£©Į½µŗĻą±Č£¬Źµ¼ŹĆ껿½Ļ“óµÄŹĒ£Ø £©µŗ”£µ±ŅŅµŗµÄĒųŹ±ĪŖ6ŌĀ9ČÕ6Ź±£¬¼×µŗĖłŌŚµÄŹ±ĒųµÄĒųŹ±ĪŖ6ŌĀ£Ø £©ČÕ£Ø £©Ź±”£ĪŅ¹ś“¦ŌŚĀ”¶¬¼¾½Ś£¬¼×µŗŹ¢ŠŠ·ēĻņĪŖ£Ø £©·ē”£
£Ø3£©ŅŅµŗÖ÷ŅŖŹĒÓÉ£Ø £©£ØÄŚ»ņĶā£©Į¦×÷ÓĆŠĪ³ÉµÄ£¬µŲŠĪŅŌ£Ø £©ĪŖÖ÷£¬µŲŹĘĢŲµćŹĒ£Ø £©”£
£Ø4£©¼×”¢ŅŅĮ½µŗÖŠ£¬¹«Ā·ĆÜ¶Č½ĻµĶµÄŹĒ£Ø £©µŗ£¬µ¼ÖĀøƵŗ¹«Ā·ĆÜ¶Č½ĻµĶµÄÖ÷ŅŖ×ŌČ»ŌŅņŹĒ£Ø £©”£
£Ø5£©ÅŠ¶Ļ¼×µŗ×ī“ó³ĒÕņĖłŌŚµŲ£¬²¢ŌŚĶ¼ÉĻ°ŃøĆ³ĒÕņµÄ·ūŗĻȦ³öĄ“£»²¢ĖµĆ÷ÅŠ¶ĻµÄĄķÓÉ”£
£Ø1£©Ī÷ Ģ«Ę½ Ó”¶Č £Ø2£©ŅŅ 8 15£ØĻĀĪē3£© ¶«ÄĻ£Ø3£©ÄŚ ɽµŲ£ØĒšĮź£© ÖŠ¼äøߣ¬ĖÄÖܵĶ£Øøß²ī“ó£¬ĘĀ¶Č¶ø£©£Ø4£©ŅŅ µŲŠĪ £Ø5£©¼×µŗĪ÷²ąÖŠ²æ½»ĶØĻß½»µć“¦ĪŖøƵŗ×ī“óµÄ³ĒÕņ£¬ČēĶ¼”£ĄķÓÉ£ŗ¢ŁĪ»ÓŚ»·µŗ¹«Ā·Óėŗį“©µŗÓģ¹«Ā·µÄ½»µć£Ø½»ĶØŹąÅ¦£©£»¢Śø½½üÓŠ»ś³”£»¢ŪµŲ“¦ŗ£±õ”£
±¾ĢāĶعżĒųÓņ¶Ō±Č“īĮŖĻµ£¬ĶعżĒųÓņ·ÖĪöæ¼²éÖŖŹ¶ŌĖÓĆ”£ŅŌĢīæÕŠĪŹ½×÷ĪŖ漲鷽Ź½£¬Ź¹ŹŌĢā¾ßÓŠ½ĻĒæµÄĒ×ŗĶĮ¦”£
µŚ£Ø1£©Ģāæ¼²éæÕ¼ä¶ØĪ»”Ŗ”Ŗ¶«Ī÷°ėĒņ¶ØĪ»£Ø20??WŗĶ160??E£©£»“óŃó¶ØĪ»£ØĄūÓĆ¾¶Č£©”£ŹĒ¶ŌµŲĄķ½ēĻߵď¶¼Ē漲锣120??WŅŌ¶«ĪŖĪ÷°ėĒņ£¬¼×µŗÖÜĪ§µÄĖ®ÓņŹōÓŚĢ«Ę½Ńó£¬56??Eø½½üĖ®ÓņĪŖÓ”¶ČŃó”£
µŚ£Ø2£©Ģāæ¼²éµŲĄķ¼ĘĖć”Ŗ”Ŗ±ČĄż³ßŗĶĒųŹ±¼ĘĖć”£¼×ŅŅĮ½Ķ¼Ķ¼·łĻąĶ¬£¬Ć껿Ļą½ü£¬µ«±ČĄż³ß¼×“óÓŚŅŅ£¬ŅŅ1cmĶ¼ÉĻ¾ąĄė“óÓŚ¼×£¬¹ŹŅŅµŗĆ껿“󔣵±ŅŅµŗ£Ø56??E£¬¶«ĖÄĒų£©µÄĒųŹ±ĪŖ6ŌĀ9ČÕ6Ź±£¬¼×µŗĖłŌŚµÄŹ±Ēų£Ø170??W£¬Ī÷Ź®Ņ»Ēų£©µÄĒųŹ±ĪŖ6ŌĀ8ČÕ15Ź±”£ĪŅ¹ś“¦ŌŚĀ”¶¬¼¾½Ś£¬¼×µŗŹÜ¶«ÄĻŠÅ·ē“ųæŲÖĘ£¬¹Ź“µ¶«ÄĻ·ē”£ÄŃ¶Č£ŗŹŹÖŠ
µŚ£Ø3£©Ģāæ¼²éÖŖŹ¶ĒØŅĘ”Ŗ”ŖµŲŠĪµŲŹĘ·ÖĪö”£ŅŅµŗÓėĪŅ¹śŗ£ÄĻµŗĄąĖĘ”£ŗÓĮ÷³Ź·ÅÉäד£¬ĖµĆ÷µŲŹĘÖŠ¼ä²æ”¢ĖÄÖܵĶ”£µŲŠĪŅŌɽµŲ»ņĒšĮźĪŖÖ÷£¬ÄŚĮ¦×÷ÓĆŠĪ³Éøßɽ»ņÅčµŲ”£ÄŃ¶Č£ŗŹŹÖŠ
µŚ£Ø4£©Ģāæ¼²éĒųĪ»·ÖĪö”Ŗ”Ŗ½»ĶØĻߏčĆÜ·ÖĪö”£ŹÜɽµŲµŲŠĪÓ°Ļģ£¬ŅŅµŗ¹«Ā·ĆÜ¶Č½ĻµĶ£¬¶ų¼×µŗĘ½ŌµŲŠĪĪŖÖ÷£¬²»ŹÜµŲŠĪĻŽÖĘ”£
µŚ£Ø5£©Ģāæ¼²é×ŪŗĻ·ÖĪö”Ŗ”Ŗ³ĒŹŠĒųĪ»·ÖĪö”£¼×µŗ×ī“ó³ĒÕņĖłŌŚµŲÓ¦øĆŹĒĒųĪ»ÓÅŹĘĆ÷ĻŌµÄµŲĒų£¬Ī÷²ąÖŠ²æ½»ĶØĻß½»µć“¦ĪŖøƵŗ×ī“óµÄ³ĒÕņ£¬»Ų“šĪŹĢāŅŖĮ¢×ćĢāĶ¼ŠÅĻ¢£¬æĘѧ¹ę·¶±ķŹö”£øƵŲĪ»ÓŚ»·µŗ¹«Ā·Óėŗį“©µŗÓģ¹«Ā·µÄ½»µć£Ø½»ĶØŹąÅ¦£©£»ø½½üÓŠ»ś³”£¬ĄūÓŚ¶ŌĶāĮŖĻµ£»µŲ“¦ŗ£±õ£¬×ŌČ»Ģõ¼žŗĆ”£



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠµŲĄķ Ą“Ō“£ŗ ĢāŠĶ£ŗ
£Ø32·Ö£©Ķ¼8ĪŖ¼×”¢ŅŅĮ½µŗĀŌĶ¼£¬ĘäÖŠ¼×µŗµŲŹĘµĶĘ½”£Ķź³ÉĻĀĮŠŅŖĒó”£
£Ø1£©°“¶«”¢Ī÷°ėĒņ»®·Ö£¬¼×µŗĪ»ÓŚ£Ø £©°ėĒņ”£¼×µŗÖÜĪ§µÄĖ®ÓņŹōÓŚ£Ø £©Ńó£¬ŅŅµŗÖÜĪ§µÄĖ®ÓņŹōÓŚ£Ø £©Ń󔣣Ø6·Ö£©
£Ø2£©Į½µŗĻą±Č£¬Źµ¼ŹĆ껿½Ļ“óµÄŹĒ£Ø £©µŗ”£µ±ŅŅµŗµÄĒųŹ±ĪŖ6ŌĀ9ČÕ6Ź±£¬¼×µŗĖłŌŚµÄŹ±ĒųµÄĒųŹ±ĪŖ6ŌĀ£Ø £©ČÕ£Ø £©Ź±”£ĪŅ¹ś“¦ŌŚĀ”¶¬¼¾½Ś£¬¼×µŗŹ¢ŠŠ·ēĻņĪŖ£Ø £©·ē”££Ø6·Ö£©
£Ø3£©ŅŅµŗÖ÷ŅŖŹĒÓÉ£Ø £©£ØÄŚ»ņĶā£©Į¦×÷ÓĆŠĪ³ÉµÄ£¬µŲŠĪŅŌ£Ø £©ĪŖÖ÷£¬µŲŹĘĢŲµćŹĒ£Ø £©”££Ø8·Ö£©
£Ø4£©¼×”¢ŅŅĮ½µŗÖŠ£¬¹«Ā·ĆÜ¶Č½ĻµĶµÄŹĒ£Ø £©µŗ£¬µ¼ÖĀøƵŗ¹«Ā·ĆÜ¶Č½ĻµĶµÄÖ÷ŅŖ×ŌČ»ŌŅņŹĒ£Ø £©”££Ø4·Ö£©
£Ø5£©ÅŠ¶Ļ¼×µŗ×ī“ó³ĒÕņĖłŌŚµŲ£¬²¢ŌŚĶ¼ÉĻ°ŃøĆ³ĒÕņµÄ·ūŗĻȦ³öĄ“£»²¢ĖµĆ÷ÅŠ¶ĻµÄĄķÓÉ”££Ø8·Ö£©
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠµŲĄķ Ą“Ō“£ŗ2011-2012ѧğŗŚĮś½Ź”Ēģ°²ČżÖŠø߶žĻĀŃ§ĘŚĘŚÄ©æ¼ŹŌµŲĄķŹŌ¾ķ£Ø“ų½āĪö£© ĢāŠĶ£ŗ×ŪŗĻĢā
Ķ¼8ĪŖ¼×”¢ŅŅĮ½µŗĀŌĶ¼£¬ĘäÖŠ¼×µŗµŲŹĘµĶĘ½”£Ķź³ÉĻĀĮŠŅŖĒó”£ 
£Ø1£©°“¶«”¢Ī÷°ėĒņ»®·Ö£¬¼×µŗĪ»ÓŚ£Ø £©°ėĒņ”£¼×µŗÖÜĪ§µÄĖ®ÓņŹōÓŚ£Ø £©Ńó£¬ŅŅµŗÖÜĪ§µÄĖ®ÓņŹōÓŚ£Ø £©Ńó”£
£Ø2£©ŅŅµŗÖ÷ŅŖŹĒÓÉ£Ø £©£ØÄŚ»ņĶā£©Į¦×÷ÓĆŠĪ³ÉµÄ£¬µŲŠĪŅŌ£Ø £©ĪŖÖ÷£¬µŲŹĘĢŲµćŹĒ£Ø £©”£
£Ø3£©¼×”¢ŅŅĮ½µŗÖŠ£¬¹«Ā·ĆÜ¶Č½ĻµĶµÄŹĒ£Ø £©µŗ£¬µ¼ÖĀøƵŗ¹«Ā·ĆÜ¶Č½ĻµĶµÄÖ÷ŅŖ×ŌČ»ŌŅņŹĒ£Ø £©”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠµŲĄķ Ą“Ō“£ŗ2013½ģŗŚĮś½Ź”ø߶žĻĀŃ§ĘŚĘŚÄ©æ¼ŹŌµŲĄķŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗ×ŪŗĻĢā
Ķ¼8ĪŖ¼×”¢ŅŅĮ½µŗĀŌĶ¼£¬ĘäÖŠ¼×µŗµŲŹĘµĶĘ½”£Ķź³ÉĻĀĮŠŅŖĒó”£

£Ø1£©°“¶«”¢Ī÷°ėĒņ»®·Ö£¬¼×µŗĪ»ÓŚ£Ø £©°ėĒņ”£¼×µŗÖÜĪ§µÄĖ®ÓņŹōÓŚ£Ø £©Ńó£¬ŅŅµŗÖÜĪ§µÄĖ®ÓņŹōÓŚ£Ø £©Ńó”£
£Ø2£©ŅŅµŗÖ÷ŅŖŹĒÓÉ£Ø £©£ØÄŚ»ņĶā£©Į¦×÷ÓĆŠĪ³ÉµÄ£¬µŲŠĪŅŌ£Ø £©ĪŖÖ÷£¬µŲŹĘĢŲµćŹĒ£Ø £©”£
£Ø3£©¼×”¢ŅŅĮ½µŗÖŠ£¬¹«Ā·ĆÜ¶Č½ĻµĶµÄŹĒ£Ø £©µŗ£¬µ¼ÖĀøƵŗ¹«Ā·ĆÜ¶Č½ĻµĶµÄÖ÷ŅŖ×ŌČ»ŌŅņŹĒ£Ø £©”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠµŲĄķ Ą“Ō“£ŗ2012Äź“ŗŗž±±Ź”ĘŚÖŠĮŖæ¼ø߶žµŲĄķŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗ×ŪŗĻĢā
Ķ¼8ĪŖ¼×”¢ŅŅĮ½µŗĀŌĶ¼£¬ĘäÖŠ¼×µŗµŲŹĘµĶĘ½”£Ķź³ÉĻĀĮŠŅŖĒó”£ £Ø10·Ö£©

£Ø1£©°“¶«”¢Ī÷°ėĒņ»®·Ö£¬¼×µŗĪ»ÓŚ£Ø £©°ėĒņ”£¼×µŗÖÜĪ§µÄĖ®ÓņŹōÓŚ£Ø £©Ńó£¬ŅŅµŗÖÜĪ§µÄĖ®ÓņŹōÓŚ£Ø £©Ńó”£
£Ø2£©Į½µŗĻą±Č£¬Źµ¼ŹĆ껿½Ļ“óµÄŹĒ£Ø £©µŗ”£µ±ŅŅµŗµÄĒųŹ±ĪŖ6ŌĀ9ČÕ6Ź±£¬¼×µŗĖłŌŚµÄŹ±ĒųµÄĒųŹ±ĪŖ6ŌĀ£Ø £©ČÕ£Ø £©Ź±”£ĪŅ¹ś“¦ŌŚĀ”¶¬¼¾½Ś£¬¼×µŗŹ¢ŠŠ·ēĻņĪŖ£Ø £©·ē”£
£Ø3£©ŅŅµŗÖ÷ŅŖŹĒÓÉ£Ø £©£ØÄŚ»ņĶā£©Į¦×÷ÓĆŠĪ³ÉµÄ£¬µŲŠĪŅŌ£Ø £©ĪŖÖ÷£¬µŲŹĘĢŲµćŹĒ£Ø £©”£
£Ø4£©¼×”¢ŅŅĮ½µŗÖŠ£¬¹«Ā·ĆÜ¶Č½ĻµĶµÄŹĒ£Ø £©µŗ£¬µ¼ÖĀøƵŗ¹«Ā·ĆÜ¶Č½ĻµĶµÄÖ÷ŅŖ×ŌČ»ŌŅņŹĒ£Ø £©”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠµŲĄķ Ą“Ō“£ŗ2007ÄźĘÕĶØøßµČѧŠ£ÕŠÉśĶ³Ņ»æ¼ŹŌĪÄ×ŪµŲĄķ²æ·Ö£ØÄžĻľķ£© ĢāŠĶ£ŗ×ŪŗĻĢā
£Ø32·Ö£©Ķ¼8ĪŖ¼×”¢ŅŅĮ½µŗĀŌĶ¼£¬ĘäÖŠ¼×µŗµŲŹĘµĶĘ½”£Ķź³ÉĻĀĮŠŅŖĒó”£

£Ø1£©°“¶«”¢Ī÷°ėĒņ»®·Ö£¬¼×µŗĪ»ÓŚ£Ø £©°ėĒņ”£¼×µŗÖÜĪ§µÄĖ®ÓņŹōÓŚ£Ø £©Ńó£¬ŅŅµŗÖÜĪ§µÄĖ®ÓņŹōÓŚ£Ø £©Ń󔣣Ø6·Ö£©
£Ø2£©Į½µŗĻą±Č£¬Źµ¼ŹĆ껿½Ļ“óµÄŹĒ£Ø £©µŗ”£µ±ŅŅµŗµÄĒųŹ±ĪŖ6ŌĀ9ČÕ6Ź±£¬¼×µŗĖłŌŚµÄŹ±ĒųµÄĒųŹ±ĪŖ6ŌĀ£Ø £©ČÕ£Ø £©Ź±”£ĪŅ¹ś“¦ŌŚĀ”¶¬¼¾½Ś£¬¼×µŗŹ¢ŠŠ·ēĻņĪŖ£Ø £©·ē”££Ø6·Ö£©
£Ø3£©ŅŅµŗÖ÷ŅŖŹĒÓÉ£Ø £©£ØÄŚ»ņĶā£©Į¦×÷ÓĆŠĪ³ÉµÄ£¬µŲŠĪŅŌ£Ø £©ĪŖÖ÷£¬µŲŹĘĢŲµćŹĒ£Ø £©”££Ø8·Ö£©
£Ø4£©¼×”¢ŅŅĮ½µŗÖŠ£¬¹«Ā·ĆÜ¶Č½ĻµĶµÄŹĒ£Ø £©µŗ£¬µ¼ÖĀøƵŗ¹«Ā·ĆÜ¶Č½ĻµĶµÄÖ÷ŅŖ×ŌČ»ŌŅņŹĒ£Ø £©”££Ø4·Ö£©
£Ø5£©ÅŠ¶Ļ¼×µŗ×ī“ó³ĒÕņĖłŌŚµŲ£¬²¢ŌŚĶ¼ÉĻ°ŃøĆ³ĒÕņµÄ·ūŗĻȦ³öĄ“£»²¢ĖµĆ÷ÅŠ¶ĻµÄĄķÓÉ”££Ø8·Ö£©
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com