
����Ŀ�����ԭ���ڻ�ѧ��ҵ���й㷺Ӧ�á���ͼ��ʾ����һ�����أ�װ�е��Һa��X��Y������缫�壬ͨ��������ֱ����Դ��������ش��������⣺
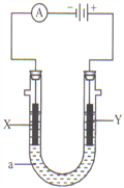
��1����X��Y���Ƕ��Ե缫��a�DZ���NaCl��Һ��ʵ�鿪ʼʱ��ͬʱ�����߸����뼸�η�̪��Һ����
��������X���ϵĵ缫��Ӧʽ�ǣ�______________����X�������۲쵽�������ǣ�__________________��
��Y�缫�ϵĵ缫��Ӧʽ�ǣ�_____________________��
��2����Ҫ�õ�ⷽ��������ͭ�����Һaѡ��CuSO4��Һ����
��X�缫�IJ�����_____��
��Y�缫�ĵ缫��Ӧʽ��_____________________�������������ʷ�Ӧ��
��3����Ҫ�����������ͭ��X�缫�IJ�����_____��
���𰸡���1��2H++2e=H2������2H2O+2e=H2��+2OH�� �ų����壬��Һ��� 2Cl 2e = Cl2��
��2����ͭ Cu2e=Cu2+
��3������
����������1����װ��ͼ��֪X���ӵ�Դ������Ϊ������������Һ�������ӵõ����ӷ�����ԭ��Ӧ�����������缫��ӦΪ��2H++2e=H2�����ƻ���ˮ�ĵ���ƽ�⣬��Һ������������Ũ������̪���ɫ�� ����X�������۲쵽�������ǣ��ų����壬��Һ��죻�� Y�缫���ӵ�Դ������Ϊ������������Һ��������ʧȥ���ӷ���������Ӧ�����������缫��ӦΪ��2Cl 2e = Cl2����
��2����Ҫ�õ�ⷽ��������ͭ�����Һaѡ��CuSO4��Һ������X���ӵ�Դ������Ϊ��Ƴص��������缫�IJ����Ǵ�ͭ����Y�缫Ϊ��Ƴص���������ͭʧȥ���Ӳ���ͭ���ӣ����������ʷ�Ӧ���缫��Ӧʽ��Cu2e=Cu2+��
��3����Ҫ�����������ͭ��X�缫�϶�ͭ��������������



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����е��� ��Դ�� ���ͣ�
����Ŀ��[2016�Ϻ�]��1��̼�H2CO3��Ki1=4.3��10-7��Ki2=5.6��10-11
���H2C2O4��Ki1=5.9��10-2��Ki2=6.4��10-5
0.1 mol/L Na2CO3��Һ��pH____________0.1 mol/L Na2C2O4��Һ��pH����ѡ��������������С����������������
��Ũ�ȵIJ�����Һ��̼����Һ�У�������Ũ�Ƚϴ����___________��
������Ũ�ȵIJ�����Һ��̼����Һ�������ϣ���Һ�и�������Ũ�ȴ�С��˳����ȷ����_____����ѡ���ţ�
a��[H+]>[HC2O4-]>[![]() ]>[
]>[![]() ]
]
b��[![]() ]>[HC2O4-]>[C2O42-]>[
]>[HC2O4-]>[C2O42-]>[![]() ]
]
c��[H+]>[HC2O4-]>[C2O42-]>[![]() ]
]
d��[H2CO3] >[![]() ]>[HC2O4-]>[
]>[HC2O4-]>[![]() ]
]
��2������ѪҺ�е�̼���̼�����δ���ƽ�⣺H++ HCO3-![]() H2CO3�������������Ի�������ʽ���ѪҺ��ʱ��ѪҺ��pH�仯������ƽ���ƶ�ԭ��������������
H2CO3�������������Ի�������ʽ���ѪҺ��ʱ��ѪҺ��pH�仯������ƽ���ƶ�ԭ��������������
____________________________________________________________________________________
�鿴�𰸺ͽ���>>
��Ŀ�����е��� ��Դ�� ���ͣ�
����Ŀ��ֻ��һ���Լ����������ȥ���������е�����(������Ϊ����)��д���Լ�����������ơ��������йصĻ�ѧ����ʽ(���ӷ�Ӧ��д���ӷ���ʽ)��
(1)Fe2O3(Al2O3)_________________________________________________��
����ʽ________________________________________________________��
(2)Fe2O3[Fe(OH)3]______________________________________________��
����ʽ_________________________________________________________��
(3)FeSO4��Һ(CuSO4)___________________________________________ __��
����ʽ__________________________________________________________��
(4)FeCl3��Һ(FeCl2)________________________________________________��
����ʽ________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����е��� ��Դ�� ���ͣ�
����Ŀ��A��B��C��D��E���ֻ����������ij�ֳ���Ԫ�أ����ǵ�ת����ϵ����ͼ��ʾ������AΪ������Һ��CΪ������ˮ�İ�ɫ���壬E��������ˮ����ȡA��Һ���գ���ɫ��ӦΪdz��ɫ(����ɫ�ܲ���)��
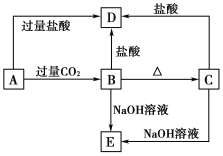
��ش��������⣺
(1)д����ѧʽ��A__________��B__________��C__________��D______��E________��
(2)д�����з�Ӧ�����ӷ���ʽ��
A��B��________________________________��
B��D��________________________________��
C��E��________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����е��� ��Դ�� ���ͣ�
����Ŀ����ҵ�����г�����NaCl��Na2SO4�����ʣ���������ͼ��ʾ��װ�òⶨ��ҵ��������Ч�ɷֵĺ�����
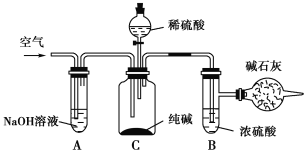
ʵ����̵���Ҫ�����ǣ�
��ȷ��ȡ��������x g(x>2)��������ƿC�С�
��ȷ����װ�м�ʯ�ҵĸ���ܵ�����Ϊy g��
���ӷ�Һ©���л���ע��ϡ���ᣬ�����ٲ�������Ϊֹ��
������������������ӣ�Ȼ�����ȡ�£�ȷ����������ΪW g��
��������ʵ�飬��д���пո�
(1)װ��A��������____________________________���������װ��A���ᵼ��ʵ����ƫ________(���С�����䡱����ͬ)��
(2)װ��B��������__________________���������װ��B���ᵼ��ʵ����ƫ________��
(3)ͨ�������������__________________________________________�������ͨ��������ᵼ��ʵ����ƫ________��
�鿴�𰸺ͽ���>>
��Ŀ�����е��� ��Դ�� ���ͣ�
����Ŀ����32.64 gͭ��140 mLһ��Ũ�ȵ����ᷴӦ��ͭ��ȫ�ܽ⣬������NO��NO2��������ڱ�״���µ����Ϊ11.2 L����ش�
(1)NO�����Ϊ________ L��NO2�����Ϊ________ L��
(2)������������ȫ���ݳ�������Һ�м���V mL a mol��L1��NaOH��Һ��ǡ��ʹ��Һ�е�Cu2+ȫ��ת���ɳ�������ԭHNO3��Һ��Ũ��Ϊ________ mol��L1��
�鿴�𰸺ͽ���>>
��Ŀ�����е��� ��Դ�� ���ͣ�
����Ŀ��
got into the room the telephone rang��
A. He hardly; then
B. Hardly had he; when
C. He had not; than
D. Not had he; when
�鿴�𰸺ͽ���>>
��Ŀ�����е��� ��Դ�� ���ͣ�
����Ŀ��ij��ѧ��ȤС��Ϊ��̽��������ij�ǽ����������γɵ�δ֪����ijɷ֣�������ͨ�����ʯ��ˮ�����ֳ���ʯ��ˮ����ǣ�����ͨ�뷢�ֻ����ֱ���壬�ɴ˸�С���Ա������ijɷ�������롣
[�������]
����1��������ΪCO2��
����2��������ΪSO2��
����3��______________________________________________________��
Ϊ����֤�²⣬��С�����ʵ�����̽����
[ʵ��̽��]
��С��ͬѧ����ͼ��ʾװ�ã��������a��ͨ�롣
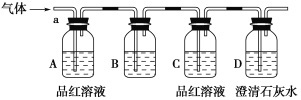
(1)B��Ӧ��װ����________�Լ�(����)��
A��NaCl��Һ
B������KMnO4��Һ
C������
D������ʯ��ˮ
(2)A��Ʒ����Һ��������________________________________________________��
(3)D�г���ʯ��ˮ��������______________________________________________��ͨ����ʵ�飬��С��ͬѧ�۲쵽��������ʵ������
��A��Ʒ����Һ��ɫ ��C��Ʒ����Һ����ɫ ��D�г���ʯ��ˮ�����
[����]
(4)����������С��ͬѧȷ�ϸ�����ijɷ�Ϊ��________________________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com