
����Ŀ�����к͵ζ����������У������¸�����������������ʵ�����á�ƫ�ߡ�����ƫ�͡�����Ӱ�족���գ�
��1���ζ���������ˮϴ����δ����֪Ũ�ȵı���Һ��ϴ��ʹ�ζ����________________��
��2����ƿ������ˮϴ�������ô�����Һ��ϴ��ʹ�ζ����________________��
��3���ζ���(װ����Һ)�ڵζ�ǰ���촦�����ݣ��ζ����������ݣ�ʹ�ζ����________________��
��4���ζ�ǰƽ�ӣ��ζ����˸��ӣ�ʹ�ζ����______________��
��5���ζ�ǰ���ӣ��ζ�����ƽ�ӣ�ʹ�ζ����______________��
��6����������յ㣬ʹ�ζ����__________________________��
��7�����������յ㣬ʹ�ζ����__________________________��
��8���ú�Na2O���ʵ�NaOH������������֪Ũ�ȵı���Һ�����ڵζ�δ֪Ũ�ȵ����ᣬʹ��������Ũ��________________��
��9���ú�Na2CO3���ʵ�NaOH������������֪Ũ�ȵı���Һ�����ڵζ�δ֪Ũ�ȵ����ᣬʹ��������Ũ��________________��
��10��ϴ����ƿʱ�����ϡʳ��ˮ��������ˮ��Ȼ������ƿװ��������ᣬ��NaOH����Һ�ζ�ʱ���Բ�õĽ��______________��
���𰸡���1��ƫ�� ��2��ƫ�� ��3��ƫ��
��4��ƫ�� ��5��ƫ�� ��6��ƫ�� ��7��ƫ�� ��8��ƫ�� ��9��ƫ�� ��10����Ӱ��
���������к͵ζ������������ݣ�c(����)=![]() ��
��![]() =
=![]() ��
��![]() ��V����
��V����
����V����ƫ���ƫС�ж�c����ƫ����ƫ�͡��磨1�����ζ���װҺǰδ�ñ���Һ��ϴ����ʹ����Һ��ϡ����������V��ƫ��ʹ�ⶨ��c����ƫ�ߡ����磨2������ƿװҺǰ�ô�����Һ��ϴ����ʹ��ƿ�ڴ�����������ӣ���������V��ƫ��ʹ�ⶨ��c����ƫ�ߣ������ѡ��ɷ´˷�����


 ͬ����ϰǿ����չϵ�д�
ͬ����ϰǿ����չϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����е��� ��Դ�� ���ͣ�
����Ŀ��(1)��Է�������Ϊ70��ϩ���ķ���ʽΪ________������ϩ����������H2�ӳɺ������ɺ�3���������������ϩ���Ŀ��ܵĽṹ��ʽΪ____________________��
(2)�л���A�Ľṹ��ʽΪ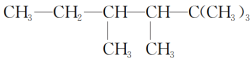
����A�ǵ�ϩ���������ӳɺ�IJ����õ�ϩ��������________�ֽṹ��
����A��Ȳ���������ӳɺ�IJ�����Ȳ��������________�ֽṹ��
����A��һ��ͬ���칹��ֻ����һ��ϩ������õ����Ҹ�ϩ����һ���dz��ԳƵķ��ӹ��ͣ���˳�������ֽṹ��
a��д��A�ĸ���ͬ���칹��Ľṹ��ʽ��_________________��
b��д������ϩ����˳�����칹��Ľṹ��ʽ��____________��
�鿴�𰸺ͽ���>>
��Ŀ�����е��� ��Դ�� ���ͣ�
����Ŀ��ijԪ��ԭ��A��L��Ҫ��M����6������,�������ֳ�����������a��b(����a�Ļ��ϼ۸���b�Ļ��ϼ�)����
(1)a��M���N��� ������;b��L���M���� �����ӡ�a���ȶ��� (����ڡ���С�ڡ�)b���ȶ��ԡ�
(2)д��Aԭ�ӵĵ����Ų�ʽ: ;a�������ĵ����Ų�ͼ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����е��� ��Դ�� ���ͣ�
����Ŀ��HIO4��Ҫ��H5IO6��ʽ���ڣ�H5IO6�ǰ�ɫ���壬������ˮ�����к�ǿ�������ԣ���ǿ����Һ��������Mn2+��
(1)����ɲ���ƽ�������ӷ�Ӧ��
__________Mn2++________H5IO6![]() __________
__________![]() +________
+________![]() +________H++________
+________H++________
(2)�����������ӷ���ʽ�ж�H5IO6��________��������(��ס����ѡ�)��
(3)������Ӧ����������Ԫ����________(��Ԫ�ط���)����1 mol Mn2+�μӷ�Ӧʱ��ת�Ƶ��ӵ����ʵ���Ϊ________��
�鿴�𰸺ͽ���>>
��Ŀ�����е��� ��Դ�� ���ͣ�
����Ŀ��(1)ά����C�ֳ�Ϊ����Ѫ�ᣬ������������Ҫ�Ĺ��ܡ����磬�������彫ʳ������ȡ�IJ������յ�Fe3+��Ϊ�����յ�Fe2+����˵��ά����C����________(������ԡ���ԭ�ԡ�)��
(2)2Na+O2![]() Na2O2��Ӧ�У�
Na2O2��Ӧ�У�
��________��������________��������������������_______��������������Ԫ�صĻ��ϼ���_______��
���˷�Ӧ����ת����2 mol�ĵ��ӣ�����Ҫ����________ mol��
���õ����Ż�˫���ŷ���ʾ��Ӧ�е���ת�Ƶķ������Ŀ��___________________________________��
(3)���ݷ�Ӧ��2FeCl3+2KI![]() 2FeCl2+2KCl+I2����2FeCl2+Cl2
2FeCl2+2KCl+I2����2FeCl2+Cl2![]() 2FeCl3���ж��������ʵ���������ǿ������˳���У���ȷ����____________��
2FeCl3���ж��������ʵ���������ǿ������˳���У���ȷ����____________��
A��Fe3+>Cl2>I2 B��Cl2>I2>Fe3+
C��I2>Cl2>Fe3+ D��Cl2>Fe3+>I2
�鿴�𰸺ͽ���>>
��Ŀ�����е��� ��Դ�� ���ͣ�
����Ŀ��������ɸ�������ɺ����ʽ��з��ࡣ
(1)��ͼ��ʾ�ķ��������________(����ĸ)��
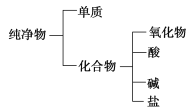
A��������෨ B����״���෨
(2)��H��O��S��N��K��Ba����Ԫ�����������ֻ�����Ԫ����ɺ��ʵij������ʣ��ֱ�����һ�ֳ������ʵĻ�ѧʽ�����±���Ӧ����У�
������� | �� | �� | �� | ������ |
��ѧʽ |
(3)���ϱ���ѡ��һ�����һ�������д���䷴Ӧ�Ļ�ѧ����ʽ��________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����е��� ��Դ�� ���ͣ�
����Ŀ��[2017����]��(As)��һЩ�����Ϳ�ɽ��ˮ�е���ȾԪ�أ�ʹ����������ȥ��ˮ�������Ч��ʩ֮һ��
��1���������̡�������������������Һ��һ���������������ʹ���ַ�Ӧ���ɻ��һ����ĸ�Ч������X��������X�к���![]() ����ԭ����___________________________________��
����ԭ����___________________________________��
��2��H3AsO3��H3AsO4ˮ��Һ�к���ĸ����ֵķֲ�����(ƽ��ʱij���ֵ�Ũ��ռ������Ũ��֮�͵ķ���)��pH�Ĺ�ϵ�ֱ�����20ͼ- 1����20ͼ- 2��ʾ��
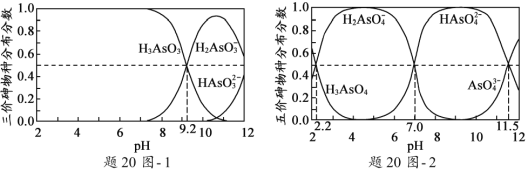
���Է�̪Ϊָʾ��(��ɫ��ΧpH 8.0 ~ 10.0)����NaOH��Һ��μ��뵽H3AsO3��Һ��������Һ����ɫ��Ϊdz��ɫʱֹͣ�μӡ��ù�������Ҫ��Ӧ�����ӷ���ʽΪ_____________________��
��H3AsO4��һ�����뷽��ʽH3AsO4![]() H2AsO4+H+�ĵ��볣��ΪKa1����pKa1=_________(pKa1=lgKa1)��
H2AsO4+H+�ĵ��볣��ΪKa1����pKa1=_________(pKa1=lgKa1)��
��3����Һ��pH��������X�������������Ӱ�졣pH =7.1ʱ��������X���治����ɣ�pH > 7.1ʱ������ɣ�pHԽ�ߣ��������������Խ�ࣻpH<7.1ʱ������ɣ�pHԽ�ͣ��������������Խ�ࡣpH��ͬʱ������X���������������ƽ��������(������ƽ��ʱ��λ����������X�����������)����20ͼ3��ʾ��
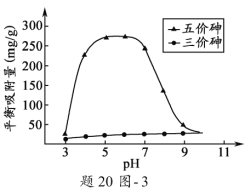
����pH7~9֮�䣬������X��������ƽ����������pH���߶�Ѹ���½�����ԭ����____________________��
����pH4~7֮�䣬������X��ˮ���������ȥ������Զ������������������Ϊ___________�� ���������X��������ȥ��Ч���ɲ�ȡ�Ĵ�ʩ��____________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����е��� ��Դ�� ���ͣ�
����Ŀ���±��Ǽ��ֳ���ȼ��(1 mol)��ȫȼ��ʱ�ų���������
���� | ̿��(C) | һ����̼(CO) | ����(H2) | ����(CH4) | �Ҵ�(C2H5OH) |
״̬ | ���� | ���� | ���� | ���� | Һ�� |
����(kJ) | 392.8 | 282.6 | 285.8 | 890.3 | 1 367 |
��1���������Ƕȷ�����Ŀǰ���ʺϼ�ͥʹ�õ���������ȼ����________��
��2��д����ʾ�ܵ�ú���е�һ����̼ȼ���ȵ��Ȼ�ѧ����ʽ_________________________��
��3�����ȼ��1 mol���и���ȼ�ϣ��ŷų�������̼����������________��
��4������ȼ�ϴ������ޣ�������ȼ�չ����л������Ⱦ��������Դ�������ķ�չս�ԣ��ҹ��������õ���ɫ��Դ�����ܡ�________�ȡ�
�鿴�𰸺ͽ���>>
��Ŀ�����е��� ��Դ�� ���ͣ�
����Ŀ���Ķ����ϣ��ش��������⡣
���ϣ����������Ƽ���Ա�о��õ�һ�����Ͳ��ϡ�����ĭ�������ǰѷ��ݼ��ӵ��������Ͻ����Ƴɵģ����ŵ���Ӳ�ȸߣ��ܶ�С(ԼΪ0.16��0.5 g/cm3)����ľ�Ļ��ᣬ�ɸ���ˮ�棬���кܴ���ԣ��Ҹ��������£���һ�����õĽ������Ϻ����ʲ��ϣ�������ɴ�����Ͷ���г���
(1)���й�����ĭ����˵���������________��
A����ĭ��������������ĭ
B����ĭ����һ�ֺϽ�
C����ĭ����һ�����ʵĽ������Ϻ����ʲ���
D����ĭ�������ڷɻ�����
(2)���Ƴ�������ʳƷ��װ��������������һ����________��
A���������� B����չ��
C�������� D��������
(3)���ڿ����лᱻ��������һ�����ܵ�����Ĥ(������)�������𱣻����ã����������Ĥ(������)����ǿ���ǿ����ܽ⣬��д����
�����ᷴӦ�����ӷ���ʽ��__________________________________________��
������������Һ��Ӧ�����ӷ���ʽ��__________________________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com