
����Ŀ���Ȼ����dz�����ˮ��������ij�Ȼ�����FeCl36H2O����Ʒ��������FeCl2���ʡ���Ҫ�ⶨ����FeCl36H2O������������ʵ�鰴���²�����У�

��֪�й����ӷ���ʽΪ��2Fe3++2I-�T2Fe2++I2��I2+2S2O32-�T2I-+S4O62-
��1��ȡ�����Ȼ�����Ʒ����50mL��ˮ�У�����Ƭ�̣�Һ����ֺ��ɫ����Ӧ�����ӷ���ʽΪ��________________________��
��2������I���õ��IJ����������ձ����������⣬��������________��_________�����������ƣ���
��3������II�����õ���������________��ѡ���ţ���
a��50mL�ձ� b��10mL��Ͳ c��20mL��Ͳ d��25mL�ζ���
ָʾ���ǵ�����Һ����ﵽ�ζ��յ��������__________________________��
��4���ζ�ʱ������Ũ��Ϊ0��1000mol/L�ı�Na2S2O3��Һ18��00mL������Ʒ��FeCl36H2O��ʽ��Ϊ270��5������������Ϊ_____________��
��5��Ҫ����Ʒ�Ȼ����е�����FeCl2���ʳ�ȥ�����õ��Լ���________��ѡ���ţ���
a������ b����ˮ c����ˮ d��˫��ˮ
��6������������²���ⶨ�Ȼ�����ƷԪ�صĺ��������������ա�
��������Ʒ ����ˮ�ܽ� ����������ˮ������ ������ ������ �����������к��ز�����
��ȱ�ٵ�һ��������________���ڹ���ǰ����Ҫ�����Ƿ������ȫ�������___________________���ж��Ƿ���صı���_________________________��
���𰸡�Fe3++ 3H2O![]() Fe(OH)3+ 3H+ 100mL����ƿ ��ͷ�ι� d ���һ�α�Һ����ʱ����ƿ����Һ����ɫ��Ϊ��ɫ���Ұ�����ڲ���ɫ 0.9738��97.38% bd ϴ�� ���ϲ���Һ�м����μӰ�ˮ���۲����������� �������γƵõ�������Ȼ���0.1g
Fe(OH)3+ 3H+ 100mL����ƿ ��ͷ�ι� d ���һ�α�Һ����ʱ����ƿ����Һ����ɫ��Ϊ��ɫ���Ұ�����ڲ���ɫ 0.9738��97.38% bd ϴ�� ���ϲ���Һ�м����μӰ�ˮ���۲����������� �������γƵõ�������Ȼ���0.1g
��������
��1���Ȼ���������Һ�����ˮ�м������������������壻
��2����������һ�����ʵ���Ũ�ȵ���Һ����Ҫ�������У���Ͳ����ͷ�ιܡ��ձ�����������һ����������ƿ��
��3��������������Һ����ľ�ȷ�ȿ�֪��100.00mL����Һ��Ҫ����������ȡ���������۱���ɫ�����Na2S2O3��Һ����͵ⵥ�ʷ�Ӧ����Һ��ɫ��Ϊ��ɫ�Ұ���Ӳ���ɫ��
��4�����ݷ�Ӧ�Ķ�����ϵ����õ���ע����Һ����ı仯��
��5��Ҫ����Ʒ�Ȼ����е�����FeCl2���ʳ�ȥ����Ҫ����������������������Ϊ�����ӣ�����������������������µ����ʣ�
��6������ʵ��������̷���������Ҫ���˺�ϴ�ӳ�ȥ��������ʣ������Ƿ������ȫ���������ϲ���Һ�м��백ˮ�۲��Ƿ��г������ɣ������������صı������γ���������ͬ��������0.001g��
��1��ȡ�����Ȼ�����Ʒ����50mL��ˮ�У�����Ƭ�̣�Һ����ֺ��ɫ�����ɵ��������������壬��Ӧ�����ӷ���ʽΪFe3++3H2O![]() Fe��OH��3+3H+��
Fe��OH��3+3H+��
�ʴ�ΪFe3++3H2O![]() Fe��OH��3+3H+��
Fe��OH��3+3H+��
��2��������һ�����ʵ���Ũ�ȵ���Һ����Ҫ�������У���Ͳ����ͷ�ιܡ��ձ�����������һ����������ƿ��
�ʴ�Ϊ100mL����ƿ����ͷ�ιܣ�
��3��100.00mL����Һ��Ҫ����������ȡ���ձ��Ǵ�����ȡ����Ͳֻ�ܾ�ȷ��0.1mL�������õζ��ܾ�ȷ��0.01mL��ѡ�õζ�����ȡ��Һ100.00mL����Һ���������۱���ɫ�����Na2S2O3��Һ����͵ⵥ�ʷ�Ӧ����Һ��ɫ�仯Ϊ��ɫ�Ұ���Ӳ���ɫ��˵���ﵽ��Ӧ�յ㣻
�ʴ�Ϊ��d����Һ��ɫ�仯Ϊ��ɫ�Ұ���Ӳ���ɫ��
��4��2Fe3++2I-��2Fe2++I2��I2+2S2O32-��2I-+S4O62-��2FeCl3-6H2O��2Fe3+��I2��2S2O32-���ζ�ʱ��10.00ml��Һ�еⵥ������Ũ��Ϊ0.1000mol/L�ı�Na2S2O3��Һ18.17mL��FeCl3-6H2O�����ʵ���=0.1000mol/L��0.01817L=0.001817mol������Ʒ��100.00mL��Һ������FeCl36H2O�����ʵ���Ϊ0.01817mol����������=![]() ��100%=98.3%��
��100%=98.3%��
�ʴ�Ϊ98.3%��
��5��Ҫ����Ʒ�Ȼ����е�����FeCl2���ʳ�ȥ����Ҫ����������������������Ϊ�����ӣ�����������������������µ����ʣ�
a�����ۺ������ӷ�Ӧ�����ܺ� �������ӷ�Ӧ����a�����ϣ�
b����ˮ����������������Ϊ�����ӣ��Ҳ������µ����ʣ���b���ϣ�
c����ˮ�������������ӣ��������������ӣ���c�����ϣ�
d��˫��ˮ����������������Ϊ�����ӣ��������ⱻ��ԭΪˮ�����������ʣ���d���ϣ�
��ѡbd��
��6��ʵ��������̷���������Ҫ���˺�ϴ�ӳ�ȥ��������ʣ������Ƿ������ȫ���������ϲ���Һ�м��백ˮ�۲��Ƿ��г������ɣ������������صı������γ���������ͬ��������0.1g��
�ʴ�Ϊ��ϴ�ӣ����������ڲ���Һ�У����백ˮ��Һ�۲����������ɣ�����������֤��������ȫ�����γ�����������Ȼ�������0.1g��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪����������K2SO4��MgSO4��2CaSO4��ˮ�д�������ƽ�⣺K2SO4��MgSO4��2CaSO4 (s)![]() 2Ca2����2K����Mg2����4SO42������ͬ�¶��£�K���Ľ���Ũ�����ܽ�ʱ��Ĺ�ϵ��ͼ��ʾ��������˵���������
2Ca2����2K����Mg2����4SO42������ͬ�¶��£�K���Ľ���Ũ�����ܽ�ʱ��Ĺ�ϵ��ͼ��ʾ��������˵���������

A.��ƽ���Ksp��c2(Ca2��)��c2(K��)��c(Mg2��)��c4(SO42��)B.�����ϵ�м��뱥��K2SO4��Һ���ܽ�ƽ�������ƶ� C.�����¶ȣ��ܽ���������ƽ��������Ӧ�����ƶ� D.�����ϵ�м��뱥��NaOH��Һ���ܽ�ƽ�ⲻ�����ƶ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������M���⽻����ҩ���ϳ�M��һ��·����ͼ��ʾ��

��֪������������A����Է�������Ϊ92
����R��CH2OH![]() RCHO
RCHO
III��R1-CHO+
IV��
V��
��ش��������⣺
��1��D��������_______��G�к��������ŵ�������_______��
��2����Ӧ�ڵķ�Ӧ����Ϊ_______��A�Ľṹ��ʽΪ_______��
��3��д����Ӧ�ߵĻ�ѧ����ʽ��______________________________��
��4��X�����������_______��̼ԭ�ӹ�ƽ�档
��5����H��ͬ���칹���У�ͬʱ�ܷ���ˮ�ⷴӦ��������Ӧ�ķ����廯�����У��˴Ź�����������4��壬�ҷ����֮��Ϊ1��1��2��6���л���Ľṹ��ʽΪ_________________��
��6����֪��![]() �����������̣�����Ա�����ȩΪ��Ҫԭ�Ϻϳ�ijҩ���м���
�����������̣�����Ա�����ȩΪ��Ҫԭ�Ϻϳ�ijҩ���м���![]() ��·��________________��
��·��________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij��ɫ��Һ�п��ܺ���![]() ��
��![]() ��
��![]() ��
��![]() ��
��![]() ��
��![]() ��
��![]() ��
��![]() ��
��![]() �е������֣�����Ũ�ȶ�Ϊ
�е������֣�����Ũ�ȶ�Ϊ![]() ������Һ�м��������
������Һ�м��������![]() ������Ļ����Һ���ް�ɫ�������ɡ�ijͬѧ��ȡ����ԭ��Һ����Ʋ��������ʵ�飺
������Ļ����Һ���ް�ɫ�������ɡ�ijͬѧ��ȡ����ԭ��Һ����Ʋ��������ʵ�飺

�����ԭ��Һ���ж�����ȷ����![]()
A.��ȷ��ԭ��Һ���Ƿ����![]()
B.�϶����ڵ�������![]() ��
��![]() ���Ƿ����
���Ƿ����![]() ��
��![]() ��Ҫͨ����ɫ��Ӧ��ȷ��
��Ҫͨ����ɫ��Ӧ��ȷ��
C.�϶������ڵ�������![]() ��
��![]() ��
��![]() ���Ƿ�
���Ƿ�![]() ����ʵ����֤
����ʵ����֤
D.��������![]() ��
��![]() ��Һ����
��Һ����![]() ������Ļ����Һ�������Һ�����ӵ��ж���Ӱ��
������Ļ����Һ�������Һ�����ӵ��ж���Ӱ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����������п��������ˮ��Ӧ�����߶���PH3���壨�۵�Ϊ-132�棬��ԭ��ǿ������ȼ������������ʳѬ��ɱ�档������ȫ���涨������ʳ�������PH3�ƣ��ĺ�������0.05mg��kg-1ʱ��ϸ������·����ⶨ��ʳ�в��������ﺬ����

���������̡���װ����װ�á�PH3�IJ��������ա�ת��KMnO4������Һ���������Ʊ���Һ�ζ���
��ʵ��װ�á�C��ʢ100 gԭ����D��ʢ�� 20.00 mL 1.12��10-4 mol L-1KMnO4�ܣ�H2SO4�ữ)��
��ش��������⣺
��1������C��������_________��ԭ������ȴ�ɷ�ĩ����ԭ����_____________��
��2��������ˮ��Ӧ�л�ѧ����ʽΪ_____________________________________���������װ�����������õķ�����_______________________________________��
��3��A��ʢװKMnO4��Һ�������dz�ȥ�����еĻ�ԭ�����壻B��ʢװ����ûʳ����ļ�����Һ�������������տ����е�O2����ֹ___________��ͨ�������������____________��
��4��D��PH3�����������ᣬ��������Ӧ�����ӷ���ʽΪ_________________________��
��5����D������Һת��������ƿ�У���ˮϡ����250mL��ȡ25.00mL����ƿ�У���5.0��10-5mol L-1��Na2SO3����Һ�ζ�ʣ���KMnO4��Һ�����ı�Na2SO3��Һ11.00mL�����ԭ���������PH3�ƣ��ĺ���Ϊ______mg kg-1����ԭ������________����ϸ��ϸ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij��ɫ��Һ�п��ܺ���Ba2+��Fe3+��Na+��K+��![]() ��
��![]() ��
��![]() ��Cl-��Br-��
��Cl-��Br-��![]() �е������֣�����Ũ�ȶ�Ϊ0.1mol/L��������Һ�м���BaCl2���������ᣬ�ް�ɫ�������ɡ���ȡ����ԭ��Һ����Ʋ��������ʵ�顣�����ж���ȷ����( )
�е������֣�����Ũ�ȶ�Ϊ0.1mol/L��������Һ�м���BaCl2���������ᣬ�ް�ɫ�������ɡ���ȡ����ԭ��Һ����Ʋ��������ʵ�顣�����ж���ȷ����( )

A.Ba2+��Fe3+��![]() ��
��![]() �϶������ڣ�Na+��K+�϶�����
�϶������ڣ�Na+��K+�϶�����
B.ԭ��Һ�϶�����![]() ��
��![]()
C.��������Ba(NO3)2��HNO3��Һ����BaCl2������Ļ����Һ������Һ�����ӵ��ж���Ӱ��
D.�Ƿ�![]() ������ʵ���ж�
������ʵ���ж�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪H2O2��KMnO4��NaClO��K2Cr2O7������ǿ�����ԡ�����Һ�е�Cu2����Fe2����Fe3������Ϊ�����������Һ��pH�ֱ�Ϊ6.4��9.6��3.7�����к�FeCl2���ʵ��Ȼ�ͭ����(CuCl2��2H2O)��Ϊ��ȡ������CuCl2��2H2O�����Ƚ����Ƴ�ˮ��Һ��Ȼ��ͼʾ��������ᴿ��

��ش��������⣺
��1����ʵ�����ʺϵ�������X��___(�����)��
A��K2Cr2O7 B��NaClO C��H2O2 D��KMnO4
��2������Y��____��
��3����ʵ���üӼ�������ܲ��ܴﵽĿ�ģ�___��ԭ����___��
��4����ȥFe3�����й����ӷ���ʽ��____��
��5������������Ŀ����____��
��6������ܲ���ֱ�������ᾧ�õ�CuCl2��2H2O����___��Ӧ��β���___��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���߷��Ӳ���E�ͺ�����Ϣʹ�߷���ҩ��ĺϳ���������ͼ��ʾ��

��֪��I��������Ϣʹ�߷���ҩ��ĽṹΪ�� ��
��
II��![]()
III��![]()
�Իش��������⣺
��1���ٵķ�Ӧ����Ϊ____________��G�ķ���ʽΪ____________��
��2����1 mol ��ת��Ϊ1 mol A��1 mol B����A��FeCl3��Һ��������ɫ��д��A��ϡ��Һ�����Ũ��ˮ������Ӧ�Ļ�ѧ����ʽ_____________��
��ת��Ϊ1 mol A��1 mol B����A��FeCl3��Һ��������ɫ��д��A��ϡ��Һ�����Ũ��ˮ������Ӧ�Ļ�ѧ����ʽ_____________��
��3����Ӧ��Ϊ�ӳɷ�Ӧ����B�Ľṹ��ʽΪ__________������Ϣʹ�Ľṹ��ʽΪ______________��
��4��д��������Ϣʹ�߷���ҩ������������������Һ������Ӧ�Ļ�ѧ����ʽ____________________��
��5��D�����ܶ�����ͬ״̬�¼����ܶȵ�6.25����D�и�Ԫ�ص����������ֱ�Ϊ̼60%����8%����32% ��D�ж���ͬ���칹�壬����������״���࣬���ܷ���������Ӧ��ͬ���칹����_______�֣�����˳���칹����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���˻����ֳ�Ϊά����B2���ɴٽ�������ϸ�����������������������������۾�ƣ�͡��˻��ط��ӵĽṹΪ��
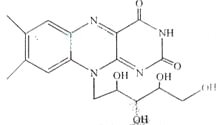
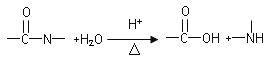
��֪��
�йغ˻��ص�����˵���У�����ȷ���ǣ�
A.�û�����ķ���ʽΪC17H22N4O6
B.���������¼���ˮ�⣬��CO2����
C.���������¼���ˮ�⣬������Һ�Ӽ����NH3����
D.�ܷ���������Ӧ
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com