
��ҵ�϶Ժ�ˮ��Դ�ۺϿ������õIJ��ֹ�����������ͼ��ʾ��
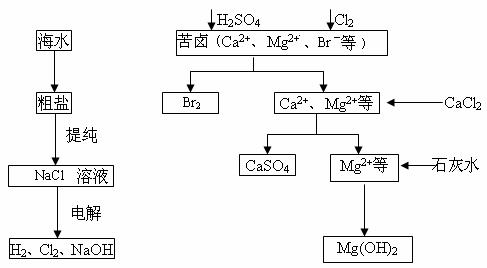
��1����ⱥ��ʳ��ˮ��������Ĥ���ۺ�Ĥ���ۡ�����Ĥ��Ĥ������ͨ���ķ��ӻ�������______________�������е���������Ϊ__________________��
��2���������������Ⱥ��Ƶ�Br2��CaSO4��Mg(OH)2���ܷ�Br2��Mg(OH)2��CaSO4��˳���Ʊ���______________________ԭ����______________________��
��3���嵥�������Ȼ�̼�е��ܽ�ȱ���ˮ�д�ö࣬���Ȼ�̼��ˮ�����ܣ��ʿ�������ȡ�壬��������������ȴ�������Ȼ�̼��ԭ����______________________��
��1�������ӣ���Na+�����ѣ���ʯī��
��2��������ȳ���Mg(OH)2��������л������CaSO4��������Ʒ������
��3�����Ȼ�̼��ȡ�����ո��ӡ��豸Ͷ�ʴ���Ч��͡�������Ⱦ���ء�
�����������ǿ����ⱥ��ʳ��ˮ������˳��Ԫ�ػ�����֪ʶ�����۱����Ϊ�����ҡ������ң����õ�����Ĥֻ��ͨ�������ӣ�����ͨ��������ӡ�CaSO4������Mg(OH)2����ǰ�����ȳ���Mg(OH)2���������һ������CaSO4��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����

| ||
| ||


 ���׳ơ����������������������谷���������ᣨ
���׳ơ����������������������谷���������ᣨ 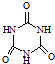 �������������������谷�����֮��ͨ��
�������������������谷�����֮��ͨ��| ������ | �ܶ�/g?cm-3 | �е�/�� | �ܽ��/100gˮ |
| ������ | 0.810 | 118.0 | 9 |
| ������ | 1.049 | 118.1 | �� |
| ���������� | 0.882 | 126.1 | 0.7 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��2008?��ɽһģ��ѡ���⣺����ѧר��ͨ��ʵ�鷢�֣��ڴ��Ե���Ӧ��λ-���������ġ���������͵ĵ������ᴦ���ƺ����ȿ��ֵ�״̬�������Ѿ������������ġ����ֵ��Ե�ͼ���Ƴ���������Ϊ���ڸ�����֮�䴫����Ϣ�Ļ�ѧ�����Ƕ�Ͱ������ԡ��������ġ��ֳ�Ϊ��Ͱ�ϵͳ����Ͱ��ṹ��ͼ����ش��������⣺
��2008?��ɽһģ��ѡ���⣺����ѧר��ͨ��ʵ�鷢�֣��ڴ��Ե���Ӧ��λ-���������ġ���������͵ĵ������ᴦ���ƺ����ȿ��ֵ�״̬�������Ѿ������������ġ����ֵ��Ե�ͼ���Ƴ���������Ϊ���ڸ�����֮�䴫����Ϣ�Ļ�ѧ�����Ƕ�Ͱ������ԡ��������ġ��ֳ�Ϊ��Ͱ�ϵͳ����Ͱ��ṹ��ͼ����ش��������⣺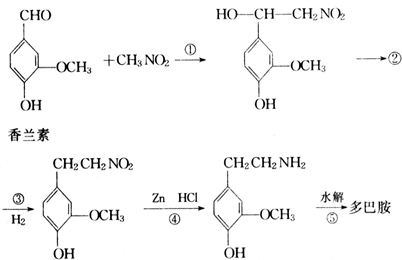




�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��������и��⣺
(1)ǰ������Ԫ���У���̬ԭ����δ�ɶԵ���������������������ͬ��Ԫ����________�֡�
(2)�ڢ�A����A��Ԫ����ɵĻ�����GaN��GaP��GaAs�����˹��ϳɵ����Ͱ뵼����ϣ��侧��ṹ�뵥�������ơ�Gaԭ�ӵĵ����Ų�ʽΪ______________����GaN�����У�ÿ��Gaԭ����__________��Nԭ����������ͬһ��Gaԭ��������Nԭ�ӹ��ɵĿռ乹��Ϊ________�����Ĵ��������У�GaN����_______���塣
(3)�ڼ��Է���NCl3�У�Nԭ�ӵĻ��ϼ�Ϊ-3,Clԭ�ӵĻ��ϼ�Ϊ+1,���Ʋ�NCl3ˮ�����Ҫ������_________(�ѧʽ)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
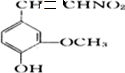
�һ����ȩ(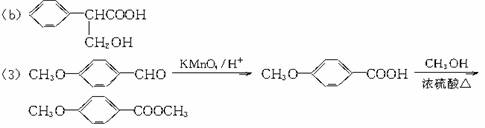 )��ʳƷ���Ӽ�������ԭ�ϣ�����ζ�����ȩ����Ũ����
)��ʳƷ���Ӽ�������ԭ�ϣ�����ζ�����ȩ����Ũ����
��1��д���һ����ȩ���������ֺ��������ŵ�����_________��
��2���һ����ȩ��ͬ���칹��A��һ���л��ᣬA�ɷ������±仯��

��ʾ����RCH2OH![]() RCHO
RCHO
���뱽��ֱ�������Ķ�̼ԭ��������ʱ����̼ԭ�Ӳſɱ�����KMnO4��Һ����Ϊ�Ȼ�
��a����A��C�ķ�Ӧ����______________���Ӧ���ͣ���
��b��д��A�Ľṹ��ʽ________________________��
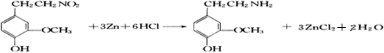
��3���һ����ȩ����һ��ͬ���칹��D��![]() ����һ��ҽҩ�м��塣����ƺ�������������ȩ��
����һ��ҽҩ�м��塣����ƺ�������������ȩ��![]() ���ϳ�D������ԭ����ѡ���÷�Ӧ����ͼ��ʾ����ע����Ҫ�ķ�Ӧ��������
���ϳ�D������ԭ����ѡ���÷�Ӧ����ͼ��ʾ����ע����Ҫ�ķ�Ӧ��������
���磬
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com