
ѧУ�����ĺ�ˮ�и�Ƽ�賤������ˮ�ʶ���ˮˮ���п��ܺ���Fe3����Ba2����K����H+��NO3����Cl-��CO32-��SO42-���ӡ�Ϊ�˽�һ��ȷ�ϣ�ȡ������ʵ���⣺
��ȡˮ����ϸ�۲죬��������һ״̬��
����pH��ֽ�ⶨ��ˮ��pH����ֽ�Ժ�ɫ��
����ˮ���е���KSCN��Һ���ʺ�ɫ��
����ˮ���е���ϡ���ᣬ�д�����ɫ�����������ټ�ϡ���ᣬ��ɫ��������ʧ��
��1���ɴ˿�֪������ˮ�п϶����е�������_________���϶�û�е�������_________��
��2�� ��Ƽ�賤�Ŀ���ԭ����ˮ�к��н϶��_____________���ӡ�
��1��Fe3����Ba2����H+�� CO32-��SO42-�� ��2��K����NO3��
���������������pH��ֽ�ⶨ��ˮ��pH����ֽ�Ժ�ɫ��˵��ˮ�������ԣ�����H+��CO32-��Ϊ����H+��Ӧ�����ڣ�������ˮ���е���KSCN��Һ���ʺ�ɫ��֤������Fe3��������CO32-��Fe3����CO32-�ᷢ�����ӷ�Ӧ����ˮ���е���ϡ���ᣬ�д�����ɫ�����������ټ�ϡ���ᣬ��ɫ��������ʧ��֤��ԭ��Һ�к���Ba2������CO32-��SO42- �����Ƕ���ͱ����ӷ�����Ӧ��ʹˮ�����ǣ�������Ŀ��ì�ܡ���ԭ��Һһ������Fe3����Ba2����H+һ������CO32-��SO42-��ֲ��������Ҫ������N��P��KԪ�أ����ˮ���������ӣ��ڸ�ˮ���и�Ƽ�賤�Ŀ���ԭ����ˮ�к��н϶��K����NO3�����ӡ�
���㣺�������ӹ��桢���ӷ�Ӧ��֪ʶ��


 ����ѧ����ϵ�д�
����ѧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ij����������ˮ��õ�����Һ�У�����Fe2+��Fe3+��SO42-��NH4+��Ba2+��CO32-�����е�ij���֡�
��1����ͬѧ��̽����Һ����ɣ�����������ʵ�飺��ȡ������Һ���Թ��У���μ���Ũ����������Һ�����ֿ�ʼ���ɰ�ɫ��������ɫ����Ѹ�ٱ�Ϊ����ɫ������Ϊ���ɫ��ͬʱ���д̼�������ų�������ȡ������Һ���Թ��У��������������ữ���ٵμ��Ȼ�����Һ���а�ɫ�������ɡ�
����Һ��һ�����е������� ��
д�����а�ɫ����ת��Ϊ���ɫ�����Ļ�ѧ����ʽ ��
��2����ͬѧ��������ʵ�飺ȡ������Һ���Թ��У��μӼ������������Һ�������������ٵμ�H2O2��������Һ���ɫ�������μ�H2O2����ɫ����ȥ�������ݲ�����ΪŪ������Ե�ɣ���ͬѧ��������֪��
H2O2��SCN-��SO42-+CO2����N2����H2O��H+
�ٸ÷�Ӧ�У���������Ԫ��Ϊ ��ÿ����lmol CO2ת�Ƶĵ�����Ϊ NA��
�ڸ�����ͬѧ��ʵ���������жϻ�ԭ��ǿ��Ϊ��Fe2+ SCN-�����=����
�۸������ϣ���ͬѧ����IJ����ǣ�H2O2��SCN-����ʹ��ɫ����ȥ���������һ��ʵ�飬��֤��ͬѧ�IJ����Ƿ���ȷ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
.����ʧȥ��ǩ����ƿ��ɫ��ҺA��B��C��D��ֻ֪��������K2CO3��K2SO4��H2SO4��Ba(NO3)2��Ϊ�˼������ǣ���������ʵ�飺
��A��D�D����Һ������ ��B��C�D����Һ������
��B��D�D����Һ������ ��A��B�D����Һ������
���ܵõ��ij���������������Һ�У����г����ܿ��ܽⲢ������ɫ��ζ����ζ��
��1����������ʵ����ʵ��A��C��D������ɫ��Һ�ֱ�Ϊ________��________��________(�û�ѧʽ��ʾ)��
��2��д���٢��з�����Ӧ�����ӷ���ʽ��
�� ��
�� ��
��3�����ӷ�Ӧ����ѧ��ѧ����Ҫ�ķ�Ӧ���ͣ��ڷ������ӷ�Ӧ�ķ�Ӧ����������У�һ��������
�ٵ��� �������� �۵���� ���� �ݻ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��һ�������������Na2CO3��Na2SO4��FeSO4��CaCl2��NaCl�Ȼ�϶��ɣ�Ϊ�������ǣ���������ʵ�飺
�� ��������������ˮ�У���������ɫ����Һ��
�� ������Һ�еμ����ᱵ��Һ���а�ɫ����������
�� ���ˣ�����������������ϡ�����У����ֳ��������ܽ⡣
��������ʵ����ʵ���ش��������⣺
��1��ԭ����������һ�����е������� ��һ�������е������� �������ѧʽ�����Կ��ܺ��е����ʣ��ɲ�������Һ�еμ� ��Һ�ķ��������顣
��2��д��������е����ӷ���ʽ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ij��Һ�н����±������е�5�����ӣ�������ˮ�ĵ��뼰���ӵ�ˮ�⣩�������ӵ����ʵ�����Ϊ1mol��
| ������ | SO42-��NO3-��Cl- |
| ������ | Fe3+��Fe2+��NH4+��Cu2+��Al3+ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
���³�ѹ�£�����10�����ʣ� ��1��Na��2��Br2��3��Na2O ��4��CO2��5��NH3��6��H2S��7��H2SO4��8��Ba(OH)2��9�� AgCl ��10������
��1���������ڵ���ʵ��� ��
��2���������ڷǵ���ʵ��� ��
��3�������ܵ������ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ij���ͭ�������ַ�ˮ��Ҫ������һ�ַ�ˮ�к���CN-���ӣ���һ�ַ�ˮ�к���Cr2O72-���ӣ��ó��ⶨ��ͼ��ʾ�ķ�ˮ�������̡�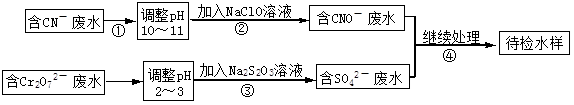 �ش��������⣺
�ش��������⣺
��1������ڷ�����Ӧ�����ӷ���ʽ�ɱ�ʾ���£�aCN��+bClO��+2cOH��=dCNO��+eN2��+fCO32��+bCl��+cH2O���������ӷ���ʽ���ܵ���ƽϵ���ж��飬��ش�
�ٷ���ʽ��e : f��ֵΪ ����ѡ���ţ���
| A��1 | B��1/2 | C��2 | D������ȷ�� |
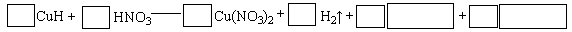
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ˮ������֮Դ��ˮ��������������������ռ����Ҫ��λ������ˮ����������ʾ��ͼ���¡�����ˮ����ƻ����������õľ�ˮ���оۺ��Ȼ�������ʽ�Ȼ����������������۱�ϩ�������ۺ��Ȼ����������Ȼ������ۺ��������ȣ�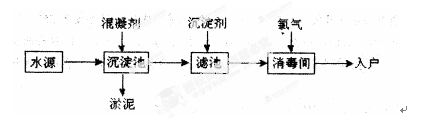
��1��FeSO4��7H2O�dz��õĻ�����������ˮ���������� ������
��2��[A12��OH��nClm]��һ�����߷��ӵĸ۾ۺϵ���ʻ�����������Ϊ�������Ȼ�������������֮���һ���м�ˮ������m��n֮��Ĺ�ϵ ��
��3��������ˮ��������������ˮ�����ʵ�飬װ����ͼ��һ��ʱ��������������ɫ���������ܿ��ɺ��ɫ��Ȼ���þ�ˮ���������ˮ�����ʵ�飻������ֻ�����������ˮ�в��������Դ���˵������ˮ�ܡ��ࡱ���������ɺ��ɫʱ�������Ļ�ѧ��Ӧ����ʽΪ�� ��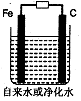
��4��MnSO4��ˮ�ʼ���е�һ�ֳ����Լ�
��MnSO4������أ�K2S2O8����������Һ�������Ӵ��¿ɷ���������ԭ��Ӧ�����ɸ�����ء������μ�����һ�ֲ��д������ƽ������Ӧ�Ļ�ѧ����ʽ�� ��
��ˮ�е��ܽ����ⶨ����Ϊ��ȡˮ����Ѹ�ټ���MnSO4��KOH���Һ���ټ���KI��Һ�������������ӣ���ʹ��ȫ��Ӧ����ӦΪ��Mn2++O2+H2O��MnOOH��δ��ƽ���������ӣ�Ѹ�ټ�������������Һ����ʱ�еⵥ�����ɡ�������ӷ�Ӧ����ʽ�� ����Na2S2O3��Һ�ζ����ɵĵ⣬����������Һ������ɼ����ˮ���ܽ���������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
��1.52 g��ͭþ�Ͻ���ȫ�ܽ���50mL14.0 mol/L��Ũ�����У��õ�NO2��N2O4�Ļ������1120mL(��״��)����Ӧ�����Һ�м���1.0 mol/L NaOH��Һ������������ȫ������ʱ���õ�2.54 g����������˵������ȷ����
| A������Ũ������ܶ�Ϊ1.40g/mL���Ũ�����������������Ϊ63% |
| B���úϽ���ͭ��þ�����ʵ���֮����2:1 |
| C��NO2��N2O4�Ļ�������У�NO2�����������80% |
| D���õ�2.54 g����ʱ������NaOH��Һ�������620 mL |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com