��������E��ˮ��Һ�е���FeCl
3��Һ����ɫ��Ӧ��˵��X�к��б����������л���X�ķ���ʽC
12H
13O
6Br�������Ͷ�Ϊ
=6�������֪���ֵĽṹ�����ж�X�����г��˱���������̼��˫���⣬û�����������ͼ���
��ת����ϵ��B������������M��M�ķ���ʽΪC
2H
2O
4����֪BΪ�Ҷ�����DΪ�Ҷ�ȩ��MΪ�Ҷ��ᣬ
���ڷ���±������ˮ�⼫�����ѣ�R
2�к���Brԭ�ӣ����R
2��Ӧ����2��̼ԭ�Ӻ�һ��-Br����-Brλ�����ˣ�����ò����Ҷ�������
�ɣ�5���п�֪��R
1�ﺬ������FeCl
3��Һ������ɫ��Ӧ�Ĺ����ţ�E�ı����ϵ�һ�ȴ���ֻ��һ�֣�����л���X�ķ���ʽ����֪���ֽṹ��֪��E�Ľṹ��ʽΪ��

�����Ͽ�֪X�Ľṹ��ʽΪ��

��G�Ľṹ��ʽΪ��

���ݴ˽��
����⣺��E��ˮ��Һ�е���FeCl
3��Һ����ɫ��Ӧ��˵��X�к��б����������л���X�ķ���ʽC
12H
13O
6Br�������Ͷ�Ϊ
=6�������֪���ֵĽṹ�����ж�X�����г��˱���������̼��˫���⣬û�����������ͼ���
��ת����ϵ��B������������M��M�ķ���ʽΪC
2H
2O
4����֪BΪ�Ҷ�����DΪ�Ҷ�ȩ��MΪ�Ҷ��ᣬ
���ڷ���±������ˮ�⼫�����ѣ�R
2�к���Brԭ�ӣ����R
2��Ӧ����2��̼ԭ�Ӻ�һ��-Br����-Brλ�����ˣ�����ò����Ҷ�������
�ɣ�5���п�֪��R
1�ﺬ������FeCl
3��Һ������ɫ��Ӧ�Ĺ����ţ�E�ı����ϵ�һ�ȴ���ֻ��һ�֣�����л���X�ķ���ʽ����֪���ֽṹ��֪��E�Ľṹ��ʽΪ��

�����Ͽ�֪X�Ľṹ��ʽΪ��

��G�Ľṹ��ʽΪ��

��
��1��MΪ�Ҷ��ᣬ�ṹ��ʽΪHOOC-COOH���׳Ʋ��ᣬ�ʴ�Ϊ��HOOC-COOH�����
��2��E�ĽṹΪ

���ṹ�к��б����ͷ��ǻ�����һ���������ܷ����ӳɡ�ȡ����������Ӧ�����ܷ�����ȥ��Ӧ���ʴ�Ϊ���ڣ�
��3��BΪ�Ҷ�����������������Hԭ�ӣ��ֱ����Ǽ����ǻ��ϣ����Ҷ������������������Ҷ�ȩ����Ӧ����ʽΪ��HOCH
2-CH
2OH+O
2OHC-CHO+2H
2O��
�ʴ�Ϊ��2��HOCH
2-CH
2OH+O
2OHC-CHO+2H
2O��
��4��GΪ

����һ�������·�����Ӧ���ɷ������ΪC
4H
4O
4���л�����л����ʹ������Ȼ�̼��Һ��ɫ�����в����ͼ�����Ϊ�ǻ�������ȥ��Ӧ��������HOOC-CH=CH-COOH����Ӧ����ʽΪHOOC-CH
2-CH��OH��-COOH
HOOC-CH=CH-COOH+H
2O��
�ʴ�Ϊ��HOOC-CH
2-CH��OH��-COOH
HOOC-CH=CH-COOH+H
2O��
��5��������������֪��X�Ľṹ��ʽΪ��

��
�ʴ�Ϊ��

��
��6��Y��G��Ϊͬ���칹�壬����ʽΪC
4H
6O
5��Y�ķ�����ֻ�����Ȼ����ǻ���ȩ�����ֹ����ţ���ͬһ��̼ԭ���ϲ���ͬʱ���������ǻ�����ṹ�к���1���Ȼ���1��ȩ����2���ǻ����ֱ�������3��̼ԭ���ϣ����ܵĽṹ��2�֣�Ϊ

���ʴ�Ϊ��2��

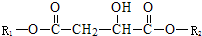 ������R1��R2Ϊδ֪���ֵĽṹ��R2�к���Brԭ�ӣ���Ϊ�Ʋ�X�ķ��ӽṹ��������ͼ��ת����
������R1��R2Ϊδ֪���ֵĽṹ��R2�к���Brԭ�ӣ���Ϊ�Ʋ�X�ķ��ӽṹ��������ͼ��ת����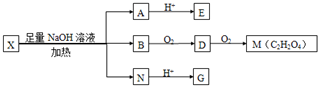


 �����Ͽ�֪X�Ľṹ��ʽΪ��
�����Ͽ�֪X�Ľṹ��ʽΪ��
 ���ݴ˽��
���ݴ˽�� �����Ͽ�֪X�Ľṹ��ʽΪ��
�����Ͽ�֪X�Ľṹ��ʽΪ��
 ��
�� ���ṹ�к��б����ͷ��ǻ�����һ���������ܷ����ӳɡ�ȡ����������Ӧ�����ܷ�����ȥ��Ӧ���ʴ�Ϊ���ڣ�
���ṹ�к��б����ͷ��ǻ�����һ���������ܷ����ӳɡ�ȡ����������Ӧ�����ܷ�����ȥ��Ӧ���ʴ�Ϊ���ڣ� ����һ�������·�����Ӧ���ɷ������ΪC4H4O4���л�����л����ʹ������Ȼ�̼��Һ��ɫ�����в����ͼ�����Ϊ�ǻ�������ȥ��Ӧ��������HOOC-CH=CH-COOH����Ӧ����ʽΪHOOC-CH2-CH��OH��-COOH
����һ�������·�����Ӧ���ɷ������ΪC4H4O4���л�����л����ʹ������Ȼ�̼��Һ��ɫ�����в����ͼ�����Ϊ�ǻ�������ȥ��Ӧ��������HOOC-CH=CH-COOH����Ӧ����ʽΪHOOC-CH2-CH��OH��-COOH ��
�� ��
�� ���ʴ�Ϊ��2��
���ʴ�Ϊ��2��


 ��ʾת�����ǣ�������
��ʾת�����ǣ�������