
( 10��) �ס������ص缫���϶���������̼��������ͼ������ش��������⣺

(1)�������о�ʢ��CuSO4��Һ����Ӧһ��ʱ���
���к�ɫ�����������ǣ��׳��е� �����ҳ��е� ����
�����ҳ��������ĵ缫��Ӧʽ�� ��
(2)�������о�ʢ�ű���NaCl��Һ��
��д���ҳ��з����ܷ�Ӧ�����ӷ���ʽ ��
�ڽ�ʪ��ĵ���KI��ֽ�����ҳظ�����������ֽ��������һ��ʱ����ַ�����ɫ��ȥ��������Ϊ������Cl2�����ɵ�I2����������Ӧ��Cl2��I2�����ʵ���֮��Ϊ5:1�������������ᡣ�÷�Ӧ�Ļ�ѧ����ʽΪ ��
�����ҳ�ת��0.02mol���Ӻ�ֹͣʵ�飬������Һ�������200mL������Һ���Ⱥ��pH= ��
(1)�� ̼(C )����(Fe) (2��)
�� 4 OH��-4 e��=== 2H2O + O2�� (2��)
(2)��2 Cl��+2H2O![]() 2 OH��+H2��+Cl2�� (2��)
2 OH��+H2��+Cl2�� (2��)
��5Cl2 + I2 + 6H2O === 10HCl + 2HIO3 (2��)
��13 (2��)
���⿼��绯����ԭ�����йؼ��㣻��1����ͼ��֪����Ϊԭ��أ���Ϊ������̼��Ϊ������ͭ������̼���ϱ���ԭ���ɿ�����ɫ������������Ϊ���أ����ݵ�������֪̼��Ϊ��������Ϊ��������Ӧһ��ʱ���ͭ��������������ԭ���ɿ����к�ɫͭ�������������ͭ�ǵ��ˮ�����ʣ������缫��ӦʽΪ��4 OH��-4 e��=== 2H2O + O2�� ��2����ⱥ��NaCl��Һ�ܷ�Ӧ�����ӷ���ʽΪ��2 Cl��+2H2O![]() 2 OH��+H2��+Cl2�� ������Ӧ��Cl2��I2�����ʵ���֮��Ϊ5:1�����ݵ�ʧ�����غ㣬��Ӧ�Ļ�ѧ����ʽΪ��5Cl2 + I2 + 6H2O === 10HCl + 2HIO3 ��
2 OH��+H2��+Cl2�� ������Ӧ��Cl2��I2�����ʵ���֮��Ϊ5:1�����ݵ�ʧ�����غ㣬��Ӧ�Ļ�ѧ����ʽΪ��5Cl2 + I2 + 6H2O === 10HCl + 2HIO3 ��
2 Cl��+2H2O![]() 2 OH��+H2��+Cl2�� ת�Ƶ���
2 OH��+H2��+Cl2�� ת�Ƶ���
2 2 mol
n(OH��) 0.02mol
n(OH��) =0.02mol
c (OH��)= =0.1mol/L
pH=13


 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��10�֡��ס��ҡ�����������������Һ���ֱ���![]() ��K+��Al3+��Ag+��Ba2+��Cl-��Br-��
��K+��Al3+��Ag+��Ba2+��Cl-��Br-��![]() ��
��![]() ��
��![]() �е�һ�����(���Ӳ��ظ�����)�����мס�������������Һ�����ԣ�����Һ�ʼ��ԣ����ҷ�Ӧ�����ɰ�ɫ���������壬���ɷֱ���ס��ҡ�����Ӧ���ɰ�ɫ����������������ʵ�ƶ�������Һ�����ʵĻ�ѧʽ��
�е�һ�����(���Ӳ��ظ�����)�����мס�������������Һ�����ԣ�����Һ�ʼ��ԣ����ҷ�Ӧ�����ɰ�ɫ���������壬���ɷֱ���ס��ҡ�����Ӧ���ɰ�ɫ����������������ʵ�ƶ�������Һ�����ʵĻ�ѧʽ��
��___________����___________����___________����___________����___________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(10��)�мס��������ݻ���ȵĺ����ܱ������������ͨ��6mol A��2mol B��������ͨ��1.5mol A��0.5mol B��3mol C�������������¶Ⱥ㶨��770K��ʹ��Ӧ3A(g)+B(g)![]() xC(g)�ﵽƽ�⣬��ʱ��üס�����������C�����������Ϊ0.2���Իش������й����⣺
xC(g)�ﵽƽ�⣬��ʱ��üס�����������C�����������Ϊ0.2���Իش������й����⣺
(1)��ƽ��ʱ���ס�����������A�����ʵ�����ȣ���x=_________��
��ƽ��ʱ���ס�����������A�����ʵ�������ȣ���x=_________��
(2)��ƽ��ʱ�������е�ѹǿ����ȣ�����������ѹǿ֮��Ϊ ��
(3)ƽ��ʱ�ס�����������A��B�����ʵ���֮���Ƿ����____________�����ȡ�����ȡ�����ƽ��ʱ����A���������Ϊ____________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��09��10�����23�и�һ��ѧ����ĩ���Ի�ѧ�� ���ͣ������
(10��)�мס��������ݻ���ȵĺ����ܱ������������ͨ��6mol A��2mol B��������ͨ��1.5mol A��0.5mol B��3mol C�������������¶Ⱥ㶨��770K��ʹ��Ӧ3A(g)+B(g) xC(g)�ﵽƽ�⣬��ʱ��üס�����������C�����������Ϊ0.2���Իش������й����⣺
xC(g)�ﵽƽ�⣬��ʱ��üס�����������C�����������Ϊ0.2���Իش������й����⣺
(1)��ƽ��ʱ���ס�����������A�����ʵ�����ȣ���x=_________��
��ƽ��ʱ���ס�����������A�����ʵ�������ȣ���x=_________��
(2)��ƽ��ʱ�������е�ѹǿ����ȣ�����������ѹǿ֮��Ϊ ��
(3)ƽ��ʱ�ס�����������A��B�����ʵ���֮���Ƿ����____________�����ȡ�����ȡ�����ƽ��ʱ����A���������Ϊ____________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ�꽭��ʡ�ϲ����и߶��ڶ����¿���ѧ�Ծ����������� ���ͣ������
(10��)�мס��������ݻ���ȵĺ����ܱ������������ͨ��6mol A��2mol B��������ͨ��1.5mol A��0.5mol B��3mol C�������������¶Ⱥ㶨��770K��ʹ��Ӧ3A(g)+B(g) xC(g)�ﵽƽ�⣬��ʱ��üס�����������C�����������Ϊ0.2���Իش������й����⣺
xC(g)�ﵽƽ�⣬��ʱ��üס�����������C�����������Ϊ0.2���Իش������й����⣺
(1)��ƽ��ʱ���ס�����������A�����ʵ�����ȣ���x= ����ƽ��ʱ���ס�����������A�����ʵ�������ȣ���x= ��
(2)ƽ��ʱ�ס�����������A��B�����ʵ���֮���Ƿ���� �����ȡ�����ȡ�����ƽ��ʱ����A���������Ϊ ��
(3)��ƽ��ʱ�������е�ѹǿ����ȣ�����������ѹǿ֮��Ϊ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013������ʡ�̽��и߶���ѧ�ڽο��Ի�ѧ�Ծ��������棩 ���ͣ������
(10��)������ס��ҡ���������ת����ϵ��
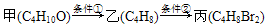
�ش�
(1)���й����ŵ�������________������________�����ʣ����ܵĽṹ��________�֣����пɴ�����Ϊȩ����________�֣�
(2)��Ӧ������Ϊ__________________________________________.
������Ϊ_______________________________________________.
(3)�ס��ҵķ�Ӧ����Ϊ________���ҡ����ķ�Ӧ����Ϊ________��
(4)���Ľṹ��ʽ��������________��
A��CH3CH2CHBrCH2Br
B��CH3CH(CH2Br)2
C��CH3CHBrCHBrCH3
D��(CH3)2CBrCH2Br
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com