
| | �� | �� | �屽 |
| �ܶ�/(g��cm��3) | 0.88 | 3.10 | 1.50 |
| �е�/�� | 80 | 59 | 156 |
| ˮ���ܽ�� | �� | �� | �� |
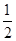 ����Һ������Ϊ15 mL��4 mL��19 mL�����ݻ�ѡ��50 mL��
����Һ������Ϊ15 mL��4 mL��19 mL�����ݻ�ѡ��50 mL��


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| ѡ�� | ʵ����� | ʵ������ | ���� |
| A | ����ҺX�м���ϡ���ᣬ������������ɫ����ͨ�����ʯ��ˮ�� | ���ɰ�ɫ���� | ��ҺX��һ������CO32����HCO3�� |
| B | ��ij��Һ���ȵμ������������ٵμ�BaCl2��Һ | �а�ɫ���� ���� | ԭ��Һ�к���SO42�� |
| C | �ֱ�ⶨͬ��ͬŨ��NaCl��CH3COONa��Һ��pH | CH3COONa��Һ��pHС | ���������ǿ�ڴ�������� |
| D | ����ˮ�Ҵ��м���ŨH2SO4��������170��C������������ͨ������KMnO4��Һ | �Ϻ�ɫ��ȥ | ʹ��Һ��ɫ�IJ�һ���� ��ϩ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| | ʵ��Ŀ�� | ʵ����� |
| A | ֤����ϩ�ܷ����ӳɷ�Ӧ | ����ϩ����ͨ��������Ȼ�̼��Һ�� |
| B | ����Fe(OH)3�����FeCl3��Һ | �ü���ʷֱ������������ʣ��Ӳ���۲��Ƿ���ֹ�����ͨ·�� |
| C | ����ƾ����Ƿ���ˮ | ȡ�����ƾ�������ˮ����ͭ��ĩ |
| D | ���𱽡�����ϩ������ | �������Ը��������Һ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A������һ��������ͬ����ֽ����ƽ���������ϣ���NaOH������������ϳ��� |
| B������ȥNO��������NO2�����������ͨ������NaOH��Һ |
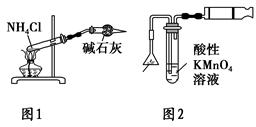
| C��ʵ������ͼ1��ʾװ����ȡ�������� |
| D��ʵ������ͼ2��ʾװ�ü�����ͷȼ�ղ�����SO2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| ѡ�� | ����(������Ϊ����) | �����Լ� | ���� |
| A | ����(NaCl) | ˮ | ���� |
| B | BaCO3(BaSO4) | ����Na2CO3��Һ | ���衢���� |
| C | ��������(����) | NaOH��Һ | ���� |
| D | �Ȼ���(Cl2) | ����ʳ��ˮ | ϴ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
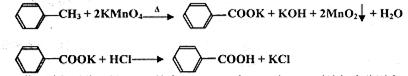
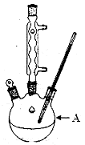

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��ˮ�����͵Ļ�����÷�Һ�������� |
| B��ʯ��ˮ�������Ĺ���̼����ù��˷������� |
| C����ˮ�еĵ��þƾ���ȡ���� |
| D���Ҵ����ܽ���ʳ�Σ����������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
| �ɷ� | NaCl | Mg(OH)2 | CaCO3 | BaSO4 | ���������� |
| ��������(��) | 15��20 | 15��20 | 5��10 | 30��40 | 10��15 |
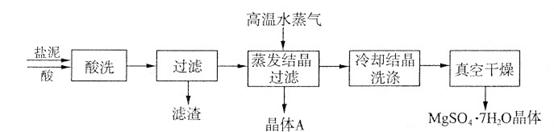
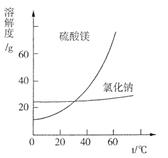
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com