

·ÖĪö øł¾ŻŌŖĖŲŌŚÖÜĘŚ±ķÖŠµÄ·Ö²¼æÉÖŖ¢Ł”«¢āŌŖĖŲ·Ö±šŹĒ£ŗH”¢C”¢N”¢O”¢Na”¢Al”¢Si”¢S”¢Cl”¢Fe£¬
£Ø1£©¢Ś¢Ü¢ŻČżÖÖŌŖĖŲ·Ö±šĪŖC”¢O”¢Na£¬ÓÉC”¢O”¢Na×é³ÉµÄĪŽ»śĪļ£ØĻą¶Ō·Ö×ÓÖŹĮæ£ŗ106£©ĪŖĢ¼ĖįÄĘ£¬Ģ¼ĖįøłĄė×Ó²æ·ÖĖ®½ā£¬ČÜŅŗĻŌŹ¾¼īŠŌ£¬¾Ż“ĖŠ“³öĢ¼ĖįøłĄė×ÓĖ®½āµÄĄė×Ó·½³ĢŹ½£»
£Ø2£©øĆ»ÆŗĻĪļĪŖĻõĖįļ§£¬ļ§øłĄė×Ó²æ·ÖĖ®½ā£¬ČÜŅŗĻŌŹ¾ĖįŠŌ£¬Č»ŗó½įŗĻµēŗÉŹŲŗćÅŠ¶Ļø÷Ąė×ÓÅØ¶Č“óŠ”£»
£Ø3£©ŌŖĖŲ¢ßµÄµ„ÖŹĪŖSi£¬ŌŖĖŲ¢ŻµÄ×īøßŃõ»ÆĪļµÄĖ®»ÆĪļĪŖNaOH£¬¹čÓėĒāŃõ»ÆÄĘČÜŅŗ·“Ӧɜ³É¹čĖįÄĘŗĶĒāĘų£»
£Ø4£©ŌŖĖŲ¢įĪŖCl£¬ŌŖĖŲ¢āµÄøß¼ŪĄė×ÓĪŖĢśĄė×Ó£¬¶žÕߊĪ³ÉµÄ»ÆŗĻĪļĪŖĀČ»ÆĢś£¬½«ĀČ»ÆĢśµĪČė·ŠĖ®ÖŠ£¬ĢśĄė×Ó·¢ÉśĖ®½āÉś³ÉĒāŃõ»ÆĢś½ŗĢ壻
£Ø5£©ĖŲ¢āµÄµ„ÖŹFeĪŖµē¼«£¬ÓɢܢŻ¢ą×é³ÉµÄ»ÆŗĻĪļ£ØĻą¶Ō·Ö×ÓÖŹĮæ£ŗ142£©ĮņĖįÄĘ£¬µē½āÖŠŃō¼«ĢśŹ§Č„µē×ÓÉś³ÉĒāŃõ»ÆŃĒĢś£¬Ņõ¼«Ė®µēĄėµÄĒāĄė×ӵƵ½µē×ÓÉś³ÉĒāĘų£¬¾Ż“ĖŠ“³öµē½āµÄ×Ü·½³ĢŹ½£»
£Ø6£©ÓÉŌŖĖŲ¢Ü¢Ž×é³ÉµÄŅõĄė×ÓæÉÓėŠ”ĖÕ“ņČÜŅŗ·“Ó¦£¬øĆŅõĄė×ÓĪŖĘ«ĀĮĖįøłĄė×Ó£¬Ģ¼ĖįĒāøłĄė×ÓµÄĖįŠŌ“óÓŚĒāŃõ»ÆĀĮ£¬ŌņĢ¼ĖįĒāøłĄė×ÓÓėĘ«ĀĮĖįøłĄė×Ó·“Ӧɜ³ÉĒāŃõ»ÆĀĮ³ĮµķŗĶĢ¼ĖįøłĄė×Ó£»
£Ø7£©¢Ł£ØX£©ĪŖH”¢¢Ś£ØY£©ĪŖC”¢¢Ü£ØZ£©ĪŖOŌŖĖŲ£¬ÓÉH”¢C”¢OČżÖÖŌŖĖŲµÄ»ÆŗĻĪļ£¬H»ÆŗĻ¼ŪĪŖ+1”¢OŌŖĖŲ»ÆŗĻ¼ŪĪŖ-2£¬½įŗĻĢ¼ŌŖĖŲµÄ×īøßÕż¼ŪĪŖ+4¶Ōø÷Ń”Ļī½ųŠŠÅŠ¶Ļ£»
£Ø8£©ŌŖĖŲ¢ŪµÄĒā»ÆĪļĪŖ°±Ęų£¬ŌŖĖŲ¢ÜµÄµ„ÖŹĪŖŃõĘų£¬ŌŚŅ»¶ØĢõ¼žĻĀ·¢ÉśÖĆ»»·“Ó¦£¬Ōņ·“Ӧɜ³ÉµŖĘųŗĶĖ®£¬øł¾ŻŃõ»Æ»¹Ō·“Ó¦ÖŠµē×ÓŹŲŗć¼ĘĖć£®
½ā“š ½ā£ŗøł¾ŻŌŖĖŲŌŚÖÜĘŚ±ķÖŠµÄ·Ö²¼æÉÖŖ¢Ł”«¢āŌŖĖŲ·Ö±šŹĒ£ŗH”¢C”¢N”¢O”¢Na”¢Al”¢Si”¢S”¢Cl”¢Fe£¬
£Ø1£©¢Ś¢Ü¢Ż·Ö±šĪŖC”¢O”¢Na£¬ÓÉC”¢O”¢Na×é³ÉµÄĪŽ»śĪļ£¬ĘäĻą¶Ō·Ö×ÓÖŹĮæĪŖ106£¬ŌņøĆĪŽ»śĪļĪŖĢ¼ĖįÄĘ£¬Ģ¼ĖįÄĘČÜŅŗÖŠĢ¼ĖįøłĄė×Ó²æ·ÖĖ®½ā£¬ČÜŅŗĻŌŹ¾¼īŠŌ£¬Ė®½āµÄĄė×Ó·½³ĢŹ½ĪŖ£ŗCO32-+H2O?HCO3-+OH-£»£¬
¹Ź“š°øĪŖ£ŗCO32-+H2O?HCO3-+OH-£»
£Ø2£©¢Ł¢Ū¢Ü·Ö±šĪŖH”¢N”¢O£¬Ö»ŗ¬¢Ł¢Ū¢ÜČżÖÖŌŖĖŲµÄŅ»ÖÖ³£¼ūĄė×Ó»ÆŗĻĪļÖŠ£¬ŌŖĖŲ¢ŪµÄ»ÆŗĻ¼Ū·Ö±š“¦ÓŚ×īøß¼ŪŗĶ×īµĶ¼Ū£¬øĆ»ÆŗĻĪļĪŖĻõĖįļ§£¬ļ§øłĄė×Ó²æ·ÖĖ®½ā£¬ČÜŅŗĻŌŹ¾ĖįŠŌ£¬Ōņc£ØH+£©£¾c£ØOH-£©£¬øł¾ŻµēŗÉŹŲŗćæÉÖŖ£ŗc£ØN03-£©£¾c £ØNH4+£©£¬ŌņČÜŅŗÖŠĄė×ÓÅØ¶Č“óŠ”ĪŖ£ŗc£ØN03-£©£¾c £ØNH4+£©£¾c£ØH+£©£¾c£ØOH-£©£¬
¹Ź“š°øĪŖ£ŗc£ØN03-£©£¾c £ØNH4+£©£¾c£ØH+£©£¾c£ØOH-£©£»
£Ø3£©ŌŖĖŲ¢ßµÄµ„ÖŹĪŖSi£¬ŌŖĖŲ¢ŻµÄ×īøßŃõ»ÆĪļµÄĖ®»ÆĪļĪŖNaOH£¬¹čÓėĒāŃõ»ÆÄĘČÜŅŗ·“Ӧɜ³É¹čĖįÄĘŗĶĒāĘų£¬·“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖ£ŗSi+2OH-+H2O=SiO32-+2H2”ü£¬
¹Ź“š°øĪŖ£ŗSi+2OH-+H2O=SiO32-+2H2”ü£»
£Ø4£©ŌŖĖŲ¢įÓėŌŖĖŲ¢āµÄøß¼Ū»ÆŗĻĪļĪŖĀČ»ÆĢś£¬½«ĀČ»ÆĢśµĪČė·ŠĖ®ÖŠ£¬ĢśĄė×Ó·¢ÉśĖ®½āÉś³ÉĒāŃõ»ÆĢś½ŗĢ壬·“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖ£ŗFe3++3H2O$\frac{\underline{\;\;”÷\;\;}}{\;}$Fe£ØOH£©3£Ø½ŗĢ壩+3H+£¬
¹Ź“š°øĪŖ£ŗFe3++3H2O$\frac{\underline{\;\;”÷\;\;}}{\;}$Fe£ØOH£©3£Ø½ŗĢ壩+3H+£»
£Ø5£©ŌŖĖŲ¢āµÄµ„ÖŹFeĪŖµē¼«£¬ÓɢܢŻ¢ą×é³ÉµÄ»ÆŗĻĪļ£ØĻą¶Ō·Ö×ÓÖŹĮæ£ŗ142£©ĮņĖįÄĘ£¬µē½ā¹ż³ĢÖŠ£¬Ńō¼«ĢśŹ§Č„µē×ÓÉś³ÉĒāŃõ»ÆŃĒĢś£¬Ņõ¼«Ė®µēĄėµÄĒāĄė×ӵƵ½µē×ÓÉś³ÉĒāĘų£¬µē½āµÄ×Ü»Æѧ·½³ĢŹ½ĪŖ£ŗFe+2H2O $\frac{\underline{\;µē½ā\;}}{\;}$ Fe£ØOH£©2”ż+H2”ü£¬
¹Ź“š°øĪŖ£ŗFe+2H2O $\frac{\underline{\;µē½ā\;}}{\;}$ Fe£ØOH£©2”ż+H2”ü£»
£Ø6£©ÓÉŌŖĖŲ¢Ü¢Ž×é³ÉµÄŅõĄė×ÓæÉÓėŠ”ĖÕ“ņČÜŅŗ·“Ó¦£¬øĆŅõĄė×ÓĪŖĘ«ĀĮĖįøłĄė×Ó£¬ÓÉÓŚĢ¼ĖįĒāøłĄė×ÓµÄĖįŠŌ“óÓŚĒāŃõ»ÆĀĮ£¬ŌņĢ¼ĖįĒāøłĄė×ÓÓėĘ«ĀĮĖįøłĄė×Ó·“Ӧɜ³ÉĒāŃõ»ÆĀĮ³Įµķ£¬·“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖ£ŗAlO2-+HCO3-+H20=Al£ØOH£©3”ż+CO32-£¬
¹Ź“š°øĪŖ£ŗAlO2-+HCO3-+H20=Al£ØOH£©3”ż+CO32-£»
£Ø7£©¢Ł£ØX£©ĪŖH”¢¢Ś£ØY£©ĪŖC”¢¢Ü£ØZ£©ĪŖOŌŖĖŲ£¬ÓÉH”¢C”¢OČżÖÖŌŖĖŲµÄ»ÆŗĻĪļ£¬H»ÆŗĻ¼ŪĪŖ+1”¢OŌŖĖŲ»ÆŗĻ¼ŪĪŖ-2£¬CŌŖĖŲ»ÆŗĻ¼ŪæÉŅŌĪŖ
A£®X2YZĪŖH2CO£¬ĪŖŅŅČ©£¬æÉŅŌ“ęŌŚøĆĪļÖŹ£¬¹ŹA“ķĪó£»
B£®X2YZ2ĪŖH2CO2£¬ĪŖ¼×Ėį£¬“ęŌŚøĆĪļÖŹ£¬¹ŹB“ķĪó£»
C£®X2YZ3ĪŖH2CO3£¬ĪŖĢ¼Ėį£¬“ęŌŚøĆĪļÖŹ£¬¹ŹC“ķĪó£»
D£®X2YZ4ĪŖH2CO4£¬CŌŖĖŲµÄ»ÆŗĻ¼ŪĪŖ+6£¬¶ųCµÄ×īøß»ÆŗĻ¼ŪĪŖ+4£¬ĖłŅŌ²»“ęŌŚøĆĪļÖŹ£¬¹ŹDÕżČ·£»
E£®X2Y2Z4ĪŖH2C2O4£¬ĪŖ²ŻĖį£¬»ÆŗĻ¼ŪĪŖ+2£¬“ęŌŚøĆĪļÖŹ£¬¹ŹE“ķĪó£»
¹Ź“š°øĪŖ£ŗD£»
£Ø8£©ŌŖĖŲ¢ŪµÄĒā»ÆĪļĪŖ°±Ęų£¬ŌŖĖŲ¢ÜµÄµ„ÖŹĪŖŃõĘų£¬ŌŚŅ»¶ØĢõ¼žĻĀ·¢ÉśÖĆ»»·“Ó¦£¬Ōņ·“Ó¦²śĪļĪŖµŖĘųŗĶĖ®£¬°±ĘųÖŠµŖŌŖĖŲµÄ»ÆŗĻ¼ŪĪŖ-3£¬·“Ó¦ŗóÉś³É0¼ŪµÄµŖĘų£¬»ÆŗĻ¼Ū±ä»ÆĪŖ3£¬·“Ó¦ÖŠNŌŖĖŲ»ÆŗĻ¼ŪÉżøß±»Ńõ»Æ£¬Ōņ°±ĘųĪŖ»¹Ō¼Į£»ŃõĘų·“Ó¦ŗóÉś³É±ä³É-2¼Ū£¬»ÆŗĻ¼Ū±ä»ÆĪŖ4£¬ÉčŃõ»Æ¼ĮŃõĘųµÄĪļÖŹµÄĮæĪŖx£¬»¹Ō¼Į°±ĘųµÄĪļÖŹµÄĮæĪŖy£¬øł¾Żµē×ÓŹŲŗćæÉµĆ£ŗ4x=3y£¬ÕūĄķæÉµĆ£ŗx£ŗy=3£ŗ4£¬
¹Ź“š°øĪŖ£ŗ3£ŗ4£®
µćĘĄ ±¾Ģāæ¼²éĮĖŌŖĖŲÖÜĘŚ±ķŗĶŌŖĖŲÖÜĘŚĀɵÄ×ŪŗĻÓ¦ÓĆ£¬ĢāÄæÄѶČÖŠµČ£¬ŹŌĢāÖŖŹ¶µć½Ļ¶ą”¢×ŪŗĻŠŌ½ĻĒ棬³ä·Öæ¼²éѧɜµÄ·ÖĪö”¢Ąķ½āÄÜĮ¦¼°Įé»īÓ¦ÓĆ»ł“”ÖŖŹ¶µÄÄÜĮ¦£¬ŹģĮ·ÕĘĪÕŌŖĖŲÖÜĘŚĀÉÄŚČŻ”¢ŌŖĖŲÖÜĘŚ±ķ½į¹¹ĪŖ½ā“š¹Ų¼ü£®


 ²½²½øß“ļ±ź¾ķĻµĮŠ“š°ø
²½²½øß“ļ±ź¾ķĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | NaOHČÜŅŗ | B£® | °±Ė® | C£® | AgNO3ČÜŅŗ | D£® | NaClČÜŅŗ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | pH=5.6µÄCH3COOHÓėCH3COONa»ģŗĻČÜŅŗÖŠ£ŗc£ØNa+£©£¼c£ØCH3COO-£© | |
| B£® | ½«pH=aµÄ“×ĖįĻ”ŹĶĪŖpH=a+1µÄ¹ż³ĢÖŠ£¬$\frac{{cCH}_{3}COOH}{{cH}^{+}}$±äŠ” | |
| C£® | ÅØ¶Č¾łĪŖ0.1 mol•L-1µÄCH3COOHŗĶCH3COONaČÜŅŗµČĢå»ż»ģŗĻŗó£ŗc£ØCH3COO-£©-c£ØCH3COOH£©=2c£ØH+£©-c£ØOH-£© | |
| D£® |  Ļņ10.00 mL 0.1 mol•L-1HCOOHČÜŅŗÖŠÖšµĪ¼ÓČė0.1 mol•L-1NaOHČÜŅŗ£¬ĘäpH±ä»ÆĒśĻßČēĶ¼ĖłŹ¾£ØŗöĀŌĪĀ¶Č±ä»Æ£©£¬ŌņcµćNaOHČÜŅŗµÄĢå»żŠ”ÓŚ10 mL Ļņ10.00 mL 0.1 mol•L-1HCOOHČÜŅŗÖŠÖšµĪ¼ÓČė0.1 mol•L-1NaOHČÜŅŗ£¬ĘäpH±ä»ÆĒśĻßČēĶ¼ĖłŹ¾£ØŗöĀŌĪĀ¶Č±ä»Æ£©£¬ŌņcµćNaOHČÜŅŗµÄĢå»żŠ”ÓŚ10 mL |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
 °±»łĖįŹĒµ°°×ÖŹµÄ»łŹÆ£¬øŹ°±ĖįŹĒ×ī¼ņµ„µÄ°±»łĖį£Ø½į¹¹ČēĶ¼£©£¬ŌŚøŹ°±Ėį·Ö×ÓÖŠ£¬NŌ×ÓµÄŌӻƊĪŹ½ŹĒsp3£¬ŌŚøŹ°±Ėį·Ö×ÓÖŠC”¢N”¢OŌ×ӵĵŚŅ»µēĄėÄܓӓ󵽊”µÄĖ³ŠņŹĒN£¾O£¾C£»µŖæÉŅŌŠĪ³É¶ąÖÖĄė×Ó£¬ČēN3-”¢NH2-”¢NH4+”¢N2H5+µČ£¬ĘäÖŠ£¬NH4+µÄæռ乹ŠĶŹĒÕżĖÄĆęĢ壮
°±»łĖįŹĒµ°°×ÖŹµÄ»łŹÆ£¬øŹ°±ĖįŹĒ×ī¼ņµ„µÄ°±»łĖį£Ø½į¹¹ČēĶ¼£©£¬ŌŚøŹ°±Ėį·Ö×ÓÖŠ£¬NŌ×ÓµÄŌӻƊĪŹ½ŹĒsp3£¬ŌŚøŹ°±Ėį·Ö×ÓÖŠC”¢N”¢OŌ×ӵĵŚŅ»µēĄėÄܓӓ󵽊”µÄĖ³ŠņŹĒN£¾O£¾C£»µŖæÉŅŌŠĪ³É¶ąÖÖĄė×Ó£¬ČēN3-”¢NH2-”¢NH4+”¢N2H5+µČ£¬ĘäÖŠ£¬NH4+µÄæռ乹ŠĶŹĒÕżĖÄĆęĢ壮²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ½«µāĖ®µ¹Čė·ÖŅŗĀ©¶·£¬¼ÓŹŹĮæŅŅ“¼£¬Õńµ“ŗó¾²ÖĆ£¬æɽ«µāŻĶČ”µ½ŅŅ“¼ÖŠ | |
| B£® | ½«ĀČ»ÆĢś±„ŗĶČÜŅŗÖó·Š£¬ŅŌÖĘČ”ĒāŃõ»ÆĢś½ŗĢå | |
| C£® | ijĪŽÉ«ČÜŅŗÖŠ¼ÓČė×ćĮæĻ”ŃĪĖįĪŽĆ÷ĻŌĻÖĻó£¬ŌŁµĪ¼ÓBaCl2ČÜŅŗÓŠ°×É«³ĮµķÉś³É£¬ŌņŌČÜŅŗÖŠŅ»¶Øŗ¬ÓŠSO42- | |
| D£® | ÓĆĪŽŠāĢśĖæÕŗČ”Ä³ČÜŅŗŌŚ¾Ę¾«µĘÉĻ×ĘÉÕ£¬»šŃę³Ź»ĘÉ«£¬ŌņĖµĆ÷øĆČÜŅŗÖŠŅ»¶Ø²»ŗ¬¼ŲĄė×Ó |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
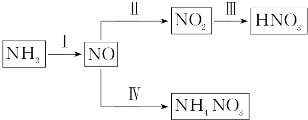
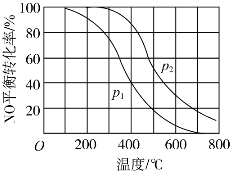
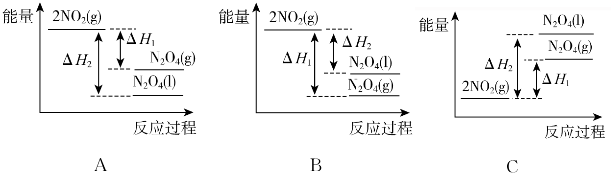
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ½«5mL0.02mol/LµÄH2SO4ČÜŅŗÓė5mL0.02mol/LNaOHČÜŅŗ³ä·Ö»ģŗĻ£¬Čō»ģŗĻŗóČÜŅŗµÄĢå»żĪŖ10mL£¬Ōņ»ģŗĻŅŗµÄpH=2 | |
| B£® | ½«0.2mol/LµÄijŅ»ŌŖĖįHAČÜŅŗŗĶ0.1mol/LNaOHČÜŅŗµČĢå»ż»ģŗĻ£¬»ģŗĻŅŗpH“óÓŚ7£¬Ōņ·“Ó¦ŗóµÄ»ģŗĻŅŗÖŠ£ŗc£ØOH-£©+c£ØA-£©£¾c£ØH+£©+c£ØHA£© | |
| C£® | pHĻąµČµÄ¢ŁCH3COONa ¢ŚC6H5ONa ¢ŪNaHCO3ČÜŅŗÖŠ£¬c£ØNa+£©“󊔹ŲĻµ£ŗ¢Ł£¾¢Ū£¾¢Ś | |
| D£® | ³£ĪĀĻĀ£¬0.1mol/LµÄCH3COONaŗĶNaClOµÄ»ģŗĻČÜŅŗÖŠ£ŗc£ØOH-£©-c£ØH+£©=c£ØHClO£©+c£ØCH3COOH£© |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ¶ąŃ”Ģā
| A£® | Y2-µÄ»¹ŌŠŌ“óÓŚZ? | B£® | µ„ÖŹµÄ»¹ŌŠŌZ£¾Y | ||
| C£® | bŅ»¶ØŠ”ÓŚc | D£® | X”¢YæÉ“¦ÓŚĶ¬ÖÜĘŚ»ņXŌŚYµÄĻĀÖÜĘŚ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
 ijČÜŅŗÖ»ŗ¬ÓŠĻĀĮŠĄė×Ó£ŗNH4+”¢Na+”¢Fe2+”¢NO3-”¢I-”¢SO32-”¢AlO2-ÖŠµÄĖÄÖÖ£ØŗöĀŌĖ®µÄµēĄė£©£¬ĒŅø÷Ąė×ÓµÄĪļÖŹµÄĮæÅضČĻąµČ£¬ĻÖ½ųŠŠČēĻĀŹµŃé£ŗ
ijČÜŅŗÖ»ŗ¬ÓŠĻĀĮŠĄė×Ó£ŗNH4+”¢Na+”¢Fe2+”¢NO3-”¢I-”¢SO32-”¢AlO2-ÖŠµÄĖÄÖÖ£ØŗöĀŌĖ®µÄµēĄė£©£¬ĒŅø÷Ąė×ÓµÄĪļÖŹµÄĮæÅضČĻąµČ£¬ĻÖ½ųŠŠČēĻĀŹµŃé£ŗ| A£® | ÓÉŹµŃé¢ŁÖ»ÄÜČ·¶ØŌČÜŅŗÖŠŅ»¶ØÓŠNH4+£¬Ć»ÓŠFe2+£® | |
| B£® | Č”ŹµŃé¢ŚŗóµÄČÜŅŗµĪ¼Óµķ·ŪČÜŅŗ£¬æÉÄܱ䥶ɫ£® | |
| C£® | ŌČÜŅŗÖŠæÉÄÜŗ¬ÓŠNH4+”¢Na+”¢SO32-”¢I-ĖÄÖÖĄė×Ó | |
| D£® | ȔɣĮæŌČÜŅŗ¼ÓĖįŠŌøßĆĢĖį¼ŲČÜŅŗ£¬æÉÄÜÓŠĮ½ÖÖĄė×Ó±»Ńõ»Æ |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com