
�ɰ�ɫ�ͺ�ɫ������ɵĻ����A�����Է������¿���ʾ��һϵ�б仯��

��1��д����Ӧ�ٵĻ�ѧ����ʽ�� ��
��Ӧ�ܵ����ӷ���ʽ�� ��
��2������ʵ��װ���п�����ʵ������ȡ����G�ķ���װ���� ������ţ���
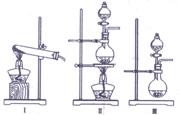
��Ϊ�˵õ��������������G���ɽ�����ͨ��a��bװ�ã�a��b�����ƿ����װ�����Һ������a�� ��b�� ��c��dװ�ò�����������ͼ�в���������

������G�ж���Ϊ�˷�ֹ��Ⱦ���������뽫β�����д�������д��d�з�����Ӧ�ĵ����ӷ���ʽ�� ��
��J��һ�ּ�������ˮ�����壬Ϊ�˷�ֹ����������e��iװ���У�����������J���� ��


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�



�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

(1)д��B���ʵĻ�ѧʽ��____________��
(2)д����Ӧ�۵Ļ�ѧ����ʽ��____________________________________���÷�Ӧ��D�����ֳ�����������________________________��
(3)�������е�������__________���ڲ���������ʹ�õIJ���������������__________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�ɰ�ɫ�ͺ�ɫ������ɵĻ����A�����Է������¿�ͼ��ʾ��һϵ�б仯��

(1)д����Ӧ�۵Ļ�ѧ����ʽ�� ��
��Ӧ�ܵ����ӷ���ʽ�� ��
(2)�����ڵ������� ���ڲ���������ʹ�õIJ��������������� ��
(3)����ʵ��װ���п�����ʵ������ȡ����G�ķ���װ���� ��Ϊ�˵õ��������������G���ɽ�����ͨ��c��dװ�ã�cװ���д�ŵ��Լ��� ��dװ���д�ŵ��Լ��� ��



(4)����G�ж���Ϊ�˷�ֹ��Ⱦ���������뽫β�����д�������д��ʵ���������ռ���Һ��������G�����ӷ���ʽ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��12�֣��ɰ�ɫ�ͺ�ɫ������ɵĻ����A�����Է������¿���ʾ��һϵ�б仯��

��1��д����Ӧ�۵Ļ�ѧ����ʽ�� ��
��Ӧ�ܵ����ӷ���ʽ�� ��
��2�������ڵ������� ��
��3������ʵ��װ���п�����ʵ������ȡ����G�ķ���װ���� ������ţ���

Ϊ�˵õ��������������G���ɽ�����ͨ��a��bװ�ã�a��b�����ƿ����װ�����Һ������a�� ��b�� ��c��dװ�ò�����������ͼ�в���������

��4������G�ж���Ϊ�˷�ֹ��Ⱦ���������뽫β�����д�������д��d�з�����Ӧ�ĵ����ӷ���ʽ�� ��
��5��J��һ�ּ�������ˮ�����壬Ϊ�˷�ֹ����������e��iװ���У�����������J��
�� ��

�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com