
ij�о�С��Ҫ������ȡ����İ������ṩ��ҩƷ�У�Ũ��ˮ���Ȼ�粒��塢�������ƹ��塢����ˮ����ʯ�ң��ṩ������������ʾ(��Ҫ�����ӡ��������ܡ��ܡ��̶�װ�ú�β������װ����ȥ)��
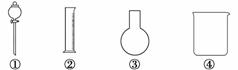
(1)��ѡ���ҩƷ��__________����С��ѡ���ҩƷ��Ϻ���ٷŰ�����ԭ��________________________________________________________________________��
ѡ���������________________(�����)��
(2)������ͼ��װ���ռ�NH3������ж���ƿ�����ռ���NH3��________________________________________________________________________��
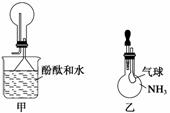
(3)ͼ���н�ͷ�ι��е�ˮ������ƿ�۲쵽��������
________________________________________________________________________��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���й����л����˵����ȷ����(����)
A����ϩ���ױ�����������е�����ԭ�Ӷ���ͬһƽ����
B����ȥ�����е���ϩʱ��ͨ���������Ӵ�������
C��C3H8�Ķ��ȴ��ﹲ��3��
D��������������ͭ���Լ������ᡢ������������Һ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����ʵ�����A(g)��B(g)���ܱ������н��з�Ӧ��A(g)��B(g)2C(g)��D(s)����H<0������������ȷ����(����)
A���ﵽƽ���Ӧ����v��(A)��2v��(C)
B���ﵽƽ�������ѹǿ��ƽ�������ƶ�
C���ﵽƽ��������¶ȣ�ƽ�������ƶ�
D��ƽ�ⳣ��KֵԽ��A��ת����Խ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����˵����ȷ���� (����)
A��Ͷ����Ƭ����H2����Һ�пɴ�������H����Mg2����SO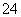 ��NO
��NO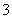
B������ϡ�����ȥ�Թ��ڱ��ϵ�����
C��1.0 mol·L��1��KNO3��Һ�пɴ�������H����Fe2����Cl����SO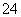
D����ͭ������������ӷ���ʽΪCuS��2H��===H2S����Cu2��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��Ҫ��д�����з�Ӧ�����ӷ���ʽ��
(1)��NH4Cl��Һ�м���NaOH��Һ��������
________________________________________________________________________��
(2)��NH4Cl��Һ�еμ�NaOH��Һ
________________________________________________________________________��
(3)NH4Cl��Һ�����Ե�ԭ��
_____________________ ___________________________________________________��
___________________________________________________��
(4)�ڱ���NH4Cl��Һ�еμ�Na[Al(OH)4]�������̼�����ζ������Ͱ�ɫ����
________________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ʵ������ȡ��������İ����漰����װ�ã�������ȷ���� (����)

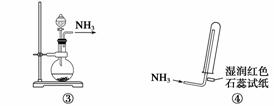
A�����ǰ�������װ�� B�����ǰ�������װ��
C�����ǰ�������װ�� D�����ǰ����ռ�������װ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����ƶ���ȷ���� (����)
A��N2��NO��NO2���Ǵ�����Ⱦ���壬�ڿ����ж����ȶ�����
B����AlCl3��Һ�еμӹ����İ�ˮ�ܿ����Ȳ�����ɫ������������ܽ�
C������Ũ�������Ũ�����ȥ�����������ͭ�Ʋ�
D����ϡ�����м���ͭ�ۣ�ͭ�۲��ܽ⣻�ټ���Cu(NO3)2���壬ͭ�۾ͻ��ܽ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�������ӷ���ʽ��д��ȷ����(����)
A��Cu(OH)2������OH����H��===H2O
B���Ƽ���ˮ�У�Na��2H2O===Na����2OH����H2��
C. FeSO2��Һ�м���ϡ���3Fe2����4H����NO ===3Fe3����2H2O��NO��
===3Fe3����2H2O��NO��
D��Al2(SO4)3��Һ�м�������Ba(OH)2��Һ��2Al3����3SO ��3Ba2����6OH��===2Al(OH)3����3BaSO4��
��3Ba2����6OH��===2Al(OH)3����3BaSO4��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com