

·ÖĪö SnCl2ŌŚŃĪĖįÖŠČܽā£¬ŌŁ¼ÓČėĪż·Ū£¬ČܽāµĆµ½SnCl2ČÜŅŗ¼ÓČėĢ¼ĖįÄĘ³ĮµķĪżĄė×Ó£¬¹żĀĖµĆµ½³ĮµķĻ“µÓŗó¼ÓČėĮņĖįČܽāµĆµ½ĮņĖįĪżČÜŅŗ£¬Õō·¢ÅØĖõĄäČ“½į¾§£¬¹żĀĖĻ“µÓµĆµ½ĮņĖįĪż¾§Ģ壬
£Ø1£©ĪżŌ×ÓµÄŗĖµēŗÉŹżĪŖ50£¬ÓėĢ¼ŌŖĖŲŹōÓŚĶ¬Ņ»Ö÷×壬“¦ÓŚ¢ōA×壬øł¾ŻŌ×ÓŠņŹż¼õø÷ÖÜĘŚČŻÄÉŌŖĖŲÖÖŹżČ·¶ØĖłŌŚµÄÖÜĘŚ£»
£Ø2£©ÓÉĮ÷³ĢĶ¼æÉÖŖ£¬²Ł×÷¢ńŹĒ“ÓČÜŅŗÖŠµĆµ½ŗ¬½į¾§Ė®µÄ¾§Ģ壬ֻÄܲÉČ”Õō·¢”¢ÅØĖõ”¢ĄäČ“½į¾§”¢¹żĀĖ”¢Ļ“µÓµĆµ½£»
£Ø3£©ÓÉŠÅĻ¢æÉÖŖ£¬SnCl2Ņ×Ė®½āÉś³É¼īŹ½ĀČ»ÆŃĒĪż£¬¼ÓČėŃĪĖį£¬ŅÖÖĘSn2+Ė®½ā£»
£Ø4£©ÓÉŠÅĻ¢æÉÖŖ£¬Sn2+Ņ×±»Ńõ»Æ£¬¼ÓČėSn·Ū³żµ÷½ŚČÜŅŗpHĶā£¬»¹·ĄÖ¹Sn2+±»Ńõ»Æ£»
£Ø5£©·“Ó¦¢ńµĆµ½³ĮµķŹĒSnO£¬SnŌŖĖŲ»ÆŗĻ¼ŪĪŖ±ä»Æ£¬ŹōÓŚ·ĒŃõ»Æ»¹Ō·“Ó¦£¬Ķ¬Ź±Éś³ÉĘųĢ壬øĆĘųĢåĪŖ¶žŃõ»ÆĢ¼£»
£Ø6£©ĖįŠŌĢõ¼žĻĀ£¬SnSO4»¹æÉŅŌÓĆ×÷Ė«ŃõĖ®Č„³ż¼Į£¬Ė«ŃõĖ®ÓŠĒæŃõ»ÆŠŌ£¬½«Sn2+Ņ×±»Ńõ»ÆĪŖSn4+£¬×ŌÉķ±»»¹ŌĪŖĖ®£»
£Ø7£©øł¾Żµē×Ó×ŖŅĘŹŲŗćÓė·½³ĢŹ½æÉµĆ¹ŲĻµŹ½Sn”«Sn2+”«2Fe3+”«2Fe2+”«$\frac{1}{3}$K2Cr2O7£¬¾Ż“Ė¼ĘĖć£®
½ā“š ½ā£ŗ£Ø1£©ĪżŌŖĖŲÓėĢ¼ŌŖĖŲŹōÓŚĶ¬Ņ»Ö÷×壬“¦ÓŚ¢ōA×壬Ō×ÓŗĖµēŗÉŹżĪŖ50£¬Ōņ£ŗ50-2-8-8-18=14£¬¹ŹSn“¦ÓŚµŚĪåÖÜĘŚ£¬ŌņŌŚÖÜĘŚ±ķÖŠµÄĪ»ÖĆĪŖ£ŗµŚĪåÖÜĘŚµŚ¢ōA×壬
¹Ź“š°øĪŖ£ŗµŚĪåÖÜĘŚµŚ¢ōA×壻
£Ø2£©ÓÉĮ÷³ĢĶ¼æÉÖŖ£¬²Ł×÷¢ńŹĒ“ÓČÜŅŗÖŠµĆµ½ŗ¬½į¾§Ė®µÄ¾§Ģ壬ֻÄܲÉČ”Õō·¢ÅØĖõ”¢ĄäČ“½į¾§”¢¹żĀĖ”¢Ļ“µÓµĆµ½£¬
¹Ź“š°øĪŖ£ŗÕō·¢ÅØĖõ”¢ĄäČ“½į¾§£»
£Ø3£©ÓÉŠÅĻ¢æÉÖŖ£¬SnCl2Ņ×Ė®½āÉś³É¼īŹ½ĀČ»ÆŃĒĪż£¬“ęŌŚĘ½ŗāSn Cl2+H2O?Sn£ØOH£©Cl+HCl£¬¼ÓČėŃĪĖį£¬Ź¹øĆĘ½ŗāĻņ×óŅĘ¶Æ£¬ŅÖÖĘSn2+Ė®½ā£¬
¹Ź“š°øĪŖ£ŗSnCl2Ė®½ā£¬·¢ÉśSnCl2+H2O?Sn£ØOH£©Cl+HCl£¬¼ÓČėŃĪĖį£¬Ź¹øĆĘ½ŗāĻņ×óŅĘ¶Æ£¬ŅÖÖĘSn2+Ė®½ā£»
£Ø4£©ÓÉŠÅĻ¢æÉÖŖ£¬Sn2+Ņ×±»Ńõ»Æ£¬¼ÓČėSn·Ū³żµ÷½ŚČÜŅŗpHĶā£¬»¹·ĄÖ¹Sn2+±»Ńõ»Æ£»
¹Ź“š°øĪŖ£ŗ·ĄÖ¹Sn2+±»Ńõ»Æ£»
£Ø5£©·“Ó¦¢ńµĆµ½³ĮµķŹĒSnO£¬SnŌŖĖŲ»ÆŗĻ¼ŪĪŖ±ä»Æ£¬ŹōÓŚ·ĒŃõ»Æ»¹Ō·“Ó¦£¬Ķ¬Ź±Éś³ÉĘųĢ壬øĆĘųĢåĪŖ¶žŃõ»ÆĢ¼£¬Ąė×Ó·½³ĢŹ½ĪŖSn2++CO32-ØTSnO”ż+CO2”ü£»¹Ź“š°øĪŖ£ŗSn2++CO32-ØTSnO”ż+CO2”ü£»
£Ø6£©ĖįŠŌĢõ¼žĻĀ£¬SnSO4»¹æÉŅŌÓĆ×÷Ė«ŃõĖ®Č„³ż¼Į£¬Ė«ŃõĖ®ÓŠĒæŃõ»ÆŠŌ£¬½«Sn2+Ņ×±»Ńõ»ÆĪŖSn4+£¬×ŌÉķ±»»¹ŌĪŖĖ®£¬Ąė×Ó·½³ĢŹ½ĪŖ£ŗSn2++H2O2+2H+ØTSn4++2H2O£»
¹Ź“š°øĪŖ£ŗSn2++H2O2+2H+ØTSn4++2H2O£»
£Ø7£©ĮīĪż·ŪÖŠĪżµÄÖŹĮæ·ÖŹżĪŖx£¬Ōņ£ŗ
Sn”«Sn2+”«2Fe3+”«2Fe2+”«$\frac{1}{3}$K2Cr2O7¼ĘĖć£®
119g $\frac{1}{3}$mol
1.226g”Įx 0.100mol/L”Į0.032L
½āµĆx=93.2%£¬
¹Ź“š°øĪŖ£ŗ93.2%£®
µćĘĄ ±¾ĢāŅŌĪļÖŹÖʱøĪŖ±³¾°æ¼²éѧɜ¶Ō¹¤ŅÕĮ÷³ĢµÄĄķ½ā£¬Éę¼°ĪļÖŹµÄ·ÖĄėĢį“攢ŌĶĮĢāÄæ»ńČ”ŠÅĻ¢µÄÄÜĮ¦”¢³£ÓĆ»ÆѧÓĆÓļŹéŠ“”¢µĪ¶ØÓ¦ÓĆ¼°ĄūÓĆ¹ŲĻµŹ½½ųŠŠµÄ¼ĘĖćµČ£¬ÄѶČÖŠµČ£¬¶ŌѧɜµÄ»ł“”ÖŖŹ¶¼°Āß¼ĶĘĄķÓŠ½ĻøßµÄŅŖĒó£®


 ¼¤»īĖ¼Ī¬ÓżÓæĪĢĆĻµĮŠ“š°ø
¼¤»īĖ¼Ī¬ÓżÓæĪĢĆĻµĮŠ“š°ø »īĮ¦ŹŌ¾ķĻµĮŠ“š°ø
»īĮ¦ŹŌ¾ķĻµĮŠ“š°ø æĪæĪÓÅÄÜĮ¦ÅąÓÅ100·ÖĻµĮŠ“š°ø
æĪæĪÓÅÄÜĮ¦ÅąÓÅ100·ÖĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | $\frac{3{V}_{1}-{V}_{2}}{{V}_{1}+{V}_{2}}$ | |
| B£® | $\frac{£Ø{V}_{1}+{V}_{2}£©”Į1{0}^{-14}}{{V}_{2}-3{V}_{1}}$ | |
| C£® | $\frac{£Ø{V}_{1}{P}_{1}+{V}_{2}{P}_{2}£©”Į1{0}^{-14}}{£Ø{V}_{2}-3{V}_{1}£©{P}_{3}}$ | |
| D£® | $\frac{£Ø{V}_{1}{P}_{1}+{V}_{2}{P}_{2}£©”Į1{0}^{-14}}{£Ø3{V}_{1}-{V}_{2}£©{P}_{3}}$ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | Ģ¼ĖįĒāļ§ČÜŅŗÓė×ćĮæµÄNaOHČÜŅŗ»ģŗĻŗó¼ÓČČ£ŗNH${\;}_{4}^{+}$+OH-$\frac{\underline{\;\;”÷\;\;}}{\;}$NH3”ü+H2O | |
| B£® | Ca£ØHCO3£©2ČÜŅŗÖŠµĪ¼ÓÉŁĮæNaOHČÜŅŗ Ca2++HCO3-+OH-=CaCO3”ż+H2O | |
| C£® | NaHSO4ČÜŅŗŗĶBa£ØOH£©2ČÜŅŗ³ä·Ö·“Ó¦ŗóČÜŅŗ³ŹÖŠŠŌ£ŗBa2++OH-+H++SO42-=BaSO4”ż+H2O | |
| D£® | ĻņFe£ØOH£©2ÖŠ¼ÓČėĻ”ĻõĖį£ŗ3Fe2++4H++NO${\;}_{3}^{-}$ØT3Fe3++NO”ü+2H2O |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | 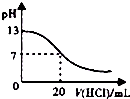 ČēĶ¼±ķŹ¾ŹŅĪĀŹ±£¬ÓĆ0.1 mol£®L-1 ŃĪĖįµĪ¶Ø0.1 mol•L-1NaOHČÜŅŗ¹ż³ĢÖŠµÄpH±ä»Æ | |
| B£® | 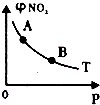 T”ꏱ£¬·“Ó¦2NO2£Øg£©  N2O4£Øg£©“ļµ½Ę½ŗāŹ±NO2µÄĢå»ż·ÖŹż¦Õ£ØNO2£©ĖęŃ¹ĒæPµÄ±ä»ÆČēĶ¼ĖłŹ¾£¬ŌņAµćµÄŃÕÉ«Éī£¬BµćµÄŃÕÉ«Ē³ N2O4£Øg£©“ļµ½Ę½ŗāŹ±NO2µÄĢå»ż·ÖŹż¦Õ£ØNO2£©ĖęŃ¹ĒæPµÄ±ä»ÆČēĶ¼ĖłŹ¾£¬ŌņAµćµÄŃÕÉ«Éī£¬BµćµÄŃÕÉ«Ē³ | |
| C£® | 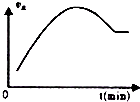 ČēĶ¼±ķŹ¾Ļņ¾ųČČŗćČŻĆܱÕČŻĘ÷ÖŠĶØČėA2ŗĶB2£¬Ņ»¶ØĢõ¼žĻĀŹ¹·“Ó¦2A2£Øg£©+B2£Øg£©?2C£Øg£©“ļµ½Ę½ŗā£¬Õż·“Ó¦ĖŁĀŹĖꏱ¼ä±ä»ÆµÄŹ¾ŅāĶ¼£®ÓÉĶ¼æÉµĆ³öµÄ½įĀŪŹĒ£ŗ·“Ó¦ĪļµÄ×ÜÄÜĮæµĶÓŚÉś³ÉĪļµÄ×ÜĮæ | |
| D£® | 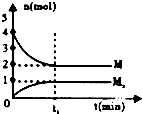 ČēĶ¼ŌŚŗćĪĀŗćČŻµÄĆܱÕČŻĘ÷ÖŠ£¬ĘųĢåM“ęŌŚČēĻĀ¹ŲĻµxM£Øg£©?Mx£Øg£©£¬t1Ź±æĢ£¬±£³ÖĪĀ¶Č²»±ä£¬ŌŁ³äČė1mol M£¬ÖŲŠĀ“ļµ½Ę½ŗāŹ±$\frac{c£ØMx£©}{c£ØM£©}$½«Ōö“ó |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
 µē½āŌĄķŌŚ»Æѧ¹¤ŅµÖŠÓŠ¹ć·ŗÓ¦ÓĆ£®ČēĶ¼±ķŹ¾Ņ»øöµē½ā³Ų£¬×°ÓŠµē½āŅŗa£»X”¢YŹĒĮ½æéµē¼«°å£¬Ķعżµ¼ĻßÓėÖ±Į÷µēŌ“ĻąĮ¬£®
µē½āŌĄķŌŚ»Æѧ¹¤ŅµÖŠÓŠ¹ć·ŗÓ¦ÓĆ£®ČēĶ¼±ķŹ¾Ņ»øöµē½ā³Ų£¬×°ÓŠµē½āŅŗa£»X”¢YŹĒĮ½æéµē¼«°å£¬Ķعżµ¼ĻßÓėÖ±Į÷µēŌ“ĻąĮ¬£®²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ¼ÓČČŹŌ¹ÜÄŚĪļÖŹŹ±£¬ŹŌ¹Üµ×²æÓė¾Ę¾«µĘĶāŃę½Ó“„ | |
| B£® | ¹żĀĖŹ±£¬Ā©¶·ĻĀ·½½ōĢłÉÕ±ÄŚ±Ś | |
| C£® | ¹żĀĖŹ±£¬²£Į§°ōÓėČż²ćĀĖÖ½µÄŅ»±ß½Ó“„ | |
| D£® | ĻņŹŌ¹ÜÖŠµĪ¼ÓŅŗĢåŹ±£¬½ŗĶ·µĪ¹Ü½ōĢłŹŌ¹ÜÄŚ±Ś |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com