

���� ��1����ͼ��֪���������ƣ�
��2��װ��I�������Ȼ�̼�;ƾ��Ļ�����Ҫ���ȣ���ˮ�½��ϳ�����ȴЧ���ã�
��3��������һ�����ʵ���Ũ�ȵ���Һ�DZ����ò��������������μӵ���̶���1��2cmʱ���ý�ͷ�ιܶ��ݣ�
��ʵ����û��450mL������ƿ��Ӧ����500mL�����m=cVM���㣻
��������Һ5000mL�����Ʋ�������ȡ��ϡ�͡���Һ��ϴ����Һ�����ݡ�ҡ�ȵȲ�����
����c=$\frac{n}{V}$��֪��nƫС��Vƫ����NaOH��ҺŨ��ƫ�ͣ��Դ������
��� �⣺��1����ͼ��֪����aΪ������ƿ���ʴ�Ϊ��������ƿ��
��2��װ��I�������Ȼ�̼�;ƾ��Ļ�����Ҫ���ȣ�ȱ������Ϊ�ƾ��ƣ��������������������ʵ�飬����ˮ��g��ͨ�룬
�ʴ�Ϊ���ƾ��ƣ�g��
��3����ȡ��ҩƷʱ��Ҫҩ�ף�����һ�����ʵ���Ũ�ȵ���Һ�DZ����ò�������������ֹҺ���⽦�����ҵ��μӵ���̶���1��2cmʱ���ý�ͷ�ιܵμ�Һ�壬
�ʴ�Ϊ��δ�ò�������������������ҩ�ס���ͷ�ιܣ�
��ʵ����û��450mL������ƿ��Ӧ����500mL������n��NaOH��=0.1mol/L��0.5L=0.05mol��m��NaOH��=0.05mol��40g/mol=2.0g��
�ʴ�Ϊ��2.0��
��������Һ5000mL�����Ʋ�������ȡ��ϡ�͡���Һ��ϴ����Һ�����ݡ�ҡ�ȵȲ�������������ƽ����2.0gNaOH�����ձ����ܽ⣬���ò��������裬��ȴ��ת�Ƶ�500mL����ƿ�У����ò�����������ϴ�ӣ�����ϴ��Һ��������ƿ������ˮ��Һ�����̶���1��2cmʱ�����ý�ͷ�ιܵμӣ�����ݵߵ�ҡ�ȣ����Բ���˳��ΪBCAFED��
�ʴ�Ϊ��BCAFED��
��A��������������ʱ����������⣬����n��С����Ũ��ƫ�ͣ���ѡ��
B��ѡ�õ�����ƿ������������ˮ����ʵ����Ӱ�죬�ʲ�ѡ��
C������ҡ�Ⱥ�Һ���½����ּ�ˮ���̶��ߣ�Vƫ����Ũ��ƫ�ͣ���ѡ��
D������ʱ���ӿ̶��ߣ�VƫС����Ũ��ƫ�ʲ�ѡ��
�ʴ�Ϊ��AC��
���� ���⿼����������ᴿ����Һ�����ƣ�Ϊ��Ƶ���㣬����������ʵ�顢ʵ�鼼�ܡ���Һ����Ϊ���Ĺؼ������ط�����ʵ�������Ŀ��飬ע��ʵ��������ܵ�ѵ������Ŀ�ѶȲ���



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��֤NH3����H+������ǿ��Ag+������NaCl��������Һ�е������ϡ���ᣬ�۲����� | |
| B�� | ����ij�������Ƿ���SO42-�������������м��������ữ��Ba��NO3��2��Һ���۲����� | |
| C�� | ����FeCl2��Һ����FeCl2���ڽ�Ũ�����У�Ȼ�������ˮϡ�ͣ��������ƺõ���Һ�м��뼸ö���������� | |
| D�� | �ᴿ�����������ı��ӣ������������ı��ӻ�����м���NaOH��Һ��������÷�Һ����ˮ������ͨ������CO2���پ��÷�Һ��ȡ�л���-���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �٢ڢۢܢ� | B�� | �ڢ� | C�� | �٢ۢܢ� | D�� | �� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 2molA 1molB | B�� | 2molA 2molB | C�� | 2molC 4molD | D�� | 2molC 2molD |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �������ȷ�Ӧ����Ӧ�������е����������������������е������� | |
| B�� | �κη��ȷ�Ӧ�ڳ�������һ���ܷ�����Ӧ | |
| C�� | ԭ��ط�Ӧһ����������ԭ��Ӧ | |
| D�� | Ԫ���ɻ���̬�������̬ʱ�������ܱ�������Ҳ���ܱ���ԭ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
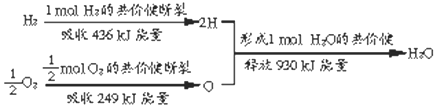
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� |  | B�� |  | C�� |  | D�� |  |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com