
 1915��ŵ��������ѧ������Henry Bragg��Lawrence Bragg���Ա���������X���߶Ծ���ṹ�ķ��������Ĺ��ף�
1915��ŵ��������ѧ������Henry Bragg��Lawrence Bragg���Ա���������X���߶Ծ���ṹ�ķ��������Ĺ��ף�| ���� | NaCl | KCl | CaO |
| ������/��kJ•mol-1�� | 786 | 715 | 3401 |
���� ��1�����Ӿ����۷е��뾧���ܳ����ȣ������������Ӱ뾶�ɷ��ȣ����ɳ����ȣ�ͬһ����Ԫ�أ����һ����������ԭ��������������������ƣ�����IIA�塢��VA��Ԫ�ص�һ�����ܴ���������Ԫ�أ�
��2����ˮ��Ϊˮ���Ӽ��������������ʹˮ���ӳ�Ϊ��ˮ���ӣ�ʹ�������С��
�ڸ��ݼ۲���ӶԻ�������ȷ�������������Sԭ���ӻ���ʽ��ԭ�Ӹ�����ȡ��۵�������ȵ�����Ϊ�ȵ����壻
�۲�ͬ�ǽ���Ԫ��֮�����γɼ��Լ������йµ��ӶԺͺ��пչ����ԭ��֮�����γ���λ����
��Cu��29��Ԫ�أ���ԭ�Ӻ�����29�����ӣ�3d��4s�ܼ�����Ϊ����Χ���ӣ�
�þ�����Cuԭ�Ӹ���=8��$\frac{1}{8}$+6��$\frac{1}{2}$=4���������=��2$\sqrt{2}$r��3cm3�����ݦ�=$\frac{m}{V}$�����ܶȣ�
��� �⣺��1�����Ӱ뾶Mg2+��Na+��O2-��Ca2+��Cl-�����ӵ����Na+=Cl-��O2-=Mg2+=Ca2+�����Ӿ�������Ӱ뾶ԽС���������Խ�࣬������Խ��������۷е�Խ�ߣ�����NaCl��KCl��MgO��CaO�۵��ɸߵ��͵�˳����MgO��CaO��NaCl��KCl��
ͬ���ڴ����ҵ�һ�����������ǵ�IIA��IIIA�塢��VA��VIA�巴�������һ������I��С���������˳����Na��Al��Mg��
�ʴ�Ϊ��MgO��CaO��NaCl��KCl��Na��Al��Mg��
��2������Ϊˮ���Ӽ��������������ʹˮ���ӳ�Ϊ��ˮ���ӣ�ʹ�������С�������������ܶȷ���õ�H2O����Է������������ۼ����������Է������ϴ�
�ʴ�Ϊ��ˮ���Ӽ���������������ã�
�������������Sԭ�Ӽ۲���ӶԸ�����4�Ҳ����µ��Ӷԣ�Sԭ���ӻ���ʽΪsp3������������ӻ�Ϊ�ȵ����������CCl4��SiCl4���ȣ�
�ʴ�Ϊ��sp3��CCl4��SiCl4���ȣ�
�۸��������N-Hԭ��֮����ڼ��Լ���Cu-Nԭ��֮�������λ������ѡAC��
��Cu��29��Ԫ�أ���ԭ�Ӻ�����29�����ӣ�3d��4s�ܼ�����Ϊ����Χ���ӣ���Χ�����Ų�ʽΪ3d104s1��
�þ�����Cuԭ�Ӹ���=8��$\frac{1}{8}$+6��$\frac{1}{2}$=4���������=��2$\sqrt{2}$r��10-10��3cm3����=$\frac{m}{V}$=$\frac{\frac{M}{{N}_{A}}��4}{V}$=$\frac{\frac{M}{{N}_{A}}��4}{��2\sqrt{2}r��1{0}^{-10}��^{3}}$g/cm3=$\frac{4M}{{{N_A}{{��2\sqrt{2}r��{{10}^{-10}}��}^3}}}$g/cm3��
�ʴ�Ϊ��3d104s1��$\frac{4M}{{{N_A}{{��2\sqrt{2}r��{{10}^{-10}}��}^3}}}$��
���� ���⿼�����ʽṹ�����ʣ�Ϊ��Ƶ���㣬�漰�������㡢ԭ���ӻ���ʽ�жϡ������ܵ�֪ʶ�㣬���ؿ���ѧ�������жϼ����������ռ������������ѵ��Ǿ������㣬ע�⣺�����������������ϵ�����ԭ����ͬһֱ���ϣ�Ϊ�״��㣮



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
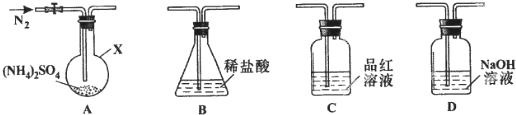
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| X | Y | ||
| Z | W | R |
| A�� | �ǽ����Ե�ǿ��˳��Y��W��R | |
| B�� | X��Y����̬�⻯����ȶ��ԣ�X��Y | |
| C�� | ԭ�Ӱ뾶�Ĵ�С˳��Z��W��Y | |
| D�� | W��R������������Ӧˮ��������ԣ�W��R |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
 ���淴ӦmA��s��+nB��g��?eC��g��+fD��g������Ӧ�����У���������������ʱ��C�İٷֺ�����C%�����¶ȣ�T����ѹǿ��p���Ĺ�ϵ��ͼ��ʾ��������������ȷ���ǣ�������
���淴ӦmA��s��+nB��g��?eC��g��+fD��g������Ӧ�����У���������������ʱ��C�İٷֺ�����C%�����¶ȣ�T����ѹǿ��p���Ĺ�ϵ��ͼ��ʾ��������������ȷ���ǣ�������| A�� | �ﵽƽ����������¶ȣ�ƽ������ | |
| B�� | �ﵽƽ��������C%���� | |
| C�� | ��ѧ����ʽ�С�n��e+f�� | |
| D�� | �ﵽƽ�������A����������ƽ�������ƶ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | pH=1����Һ��Cu2+��Na+��NO3-��SO42- | |
| B�� | �μ���ɫʯ����Һ�Ժ�ɫ����Һ��Fe2+��NH4+��Cl-��NO3- | |
| C�� | ˮ���������c��H+��=10-12mol/L����Һ��K+��Mg2+��SiO32-��Br- | |
| D�� | ����KSCN��Ѫ��ɫ����Һ�У�Na+��Ba2+��Cl-��OH- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | Ca��HCO3��2��Һ������NaOH��Һ��Ӧ��HCO3-+Ca2++OH-�TCaCO3��+H2O | |
| B�� | ��������������ϡ���3Fe2++4H++NO3-�T3Fe3++NO��+3H2O | |
| C�� | ��Al2��SO4��3��Һ�м��������NH3•H2O��Al3++4 NH3•H2O=AlO2-+4NH4++2H2O | |
| D�� | NH4HCO3���ڹ�����ŨKOH��Һ�У�NH4++HCO3-+2OH-=CO32-+NH3��+2 H2O |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com