
(1)B��N��Ӧ�����ӷ���ʽΪ��������������������������������������?
(2)����MΪ�缫���A��ˮ��Һʱ�������ϵĵ缫��ӦʽΪ�������������������������������������������ϵĵ缫��ӦʽΪ����?

(3)D��G��Ӧ����1.12 L��״���µ�Fʱ��ת�Ƶĵ�����Ϊ?��������?��?
(4)G��M��Ӧ�Ļ�ѧ����ʽΪ��������������������������������������?
(5)I�����ھ���ˮ����ԭ����(�����ӷ���ʽ��ʾ)������������������������������������������������?
(6)���Զ��Ե缫���A��ˮ��Һʱ��ͨ��һ��ʱ�������������448 mL������(��״��)����ʱ��Һ�����Ϊ400 mL������Һ��pHΪ?��������?��?
(1)Cl2 + 2OH-��Cl- + ClO-+ H2O?
(2)Fe - 2e-�� Fe2+,2H+ + 2e-��H2��?
(3)6.02��1022??
(4)4H2O(g) + 3Fe ![]() Fe3O4 + 4H2
Fe3O4 + 4H2
(5)Fe3+ + 3H2O �� Fe(OH)3(����)+ 3H+?
(6)13
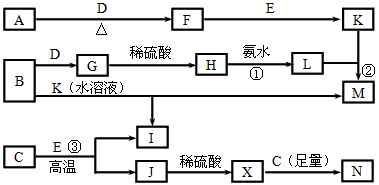
�������ɿ�ͼ��֪AΪNaCl,BΪCl2,CΪNa,DΪNa2O2,��GΪH2O��EΪH2��JΪHCl��MΪ��۽�����ΪFe,IΪFeCl3,KΪFeCl2,FΪO2��HΪFe3O4��?
(1)B��N��Cl2��NaOH��Ӧ��?
(2)�������缫���NaCl��Һ������Fe - 2e-�� Fe2+,����2H++2e-��H2����?
(3)Na2O2��H2O��Ӧ1 mol Na2O2��Ӧת�Ƶ���1 mol,1.12 L O2Ϊ0.05 mol��Na2O2 0.1 mol����ת�Ƶ���Ϊ0.1 mol��?
(4)G��M��Fe��H2O��Ӧ��4H2O(g) + 3Fe ![]() Fe3O4 + 4H2��?
Fe3O4 + 4H2��?
(5)Fe3+ˮ�����ɵ�Fe(OH)3��ˮ��?
(6)������ӦΪ��2Cl--2e-��Cl2��,448 mL Cl2Ϊ0.02 mol,��ת�Ƶ���0.04 mol,��һ�缫����0.04 mol H+��Ӧ��ͬʱ����0.04 mol OH-����c(OH-)=![]() =0.1 mol��L-1,c(H+)=1��10-13mol��L-1,pH=13��
=0.1 mol��L-1,c(H+)=1��10-13mol��L-1,pH=13��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2007����̨��ѧ������ѧ(������)�Ծ� ���ͣ�022
| |||||||||||||||||||||||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�������г�����2006��2007ѧ��ȸ����꼶��һѧ����ĩͳһ���ԡ���ѧ ���ͣ�022
| |||||||||||||||||||||||||||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��0110 ��ĩ�� ���ͣ��ƶ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
A��N��Ϊ��ѧ��ѧ�еij������ʣ�����A���ճ������в���ȱ�ٵ����ʣ�Ҳ�ǻ��������ϵ���Ҫԭ�ϣ�����M��Ŀǰʹ�������Ľ�����������B��E��FΪ���壬GΪ��ɫҺ�壬��Щ������һ�������´�������ת����ϵ��������Щ��Ӧ����������Ѿ���ȥ���ش��������⣺
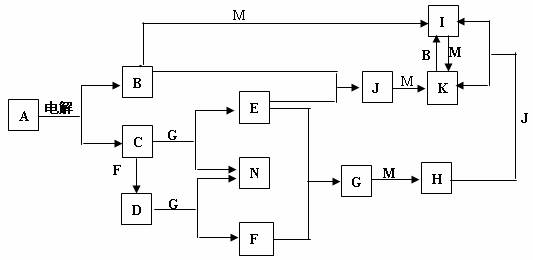
��1��H�Ļ�ѧʽΪ ��D�ĵ���ʽΪ ��
��2��Kת��ΪI�����ӷ���ʽ�� ��
��3��D��G��Ӧ�Ļ�ѧ����ʽΪ ��
��4��I�����ھ�ˮ����ԭ����
������صķ�Ӧ����ʽ�ͼ�Ҫ���ֻش𣩡�
��5��1mol����E������F����ȫȼ������Һ̬Gʱ�ų�������Ϊa kJ,��д����ʾEȼ���ȵ��Ȼ�ѧ����ʽ ��
��6��I��NaClO�ڼ��������·�Ӧ�����Ʊ��������ɫˮ��������Na2MO4����һ�ַ�������д���˷�Ӧ�Ļ�ѧ����ʽ ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com