
 ����ͼ��ʾ�����ȼ��У���100mL0.50mol•L CH3COOH��Һ ��100mL0.55mol•L-1NaOH��Һ��ϣ���֪���ȼƵ����ݳ��������ȼƸ�����ÿ����1���������������150.5J•��-1��������Һ�ı�����Ϊ4.184J•g-1•��-1����Һ���ܶȾ�����Ϊ1g•mL-1��ʵ����ijͬѧ�����к��ȣ���¼�������£�
����ͼ��ʾ�����ȼ��У���100mL0.50mol•L CH3COOH��Һ ��100mL0.55mol•L-1NaOH��Һ��ϣ���֪���ȼƵ����ݳ��������ȼƸ�����ÿ����1���������������150.5J•��-1��������Һ�ı�����Ϊ4.184J•g-1•��-1����Һ���ܶȾ�����Ϊ1g•mL-1��ʵ����ijͬѧ�����к��ȣ���¼�������£�| ʵ����� | ��ʼ�¶�t1/�� | ��ֹ�¶�t2/�� | |
| ������Һ | ����������Һ | �����Һ | |
| 1 | 25.0 | 25.1 | 27.8 |
| 2 | 25.1 | 25.3 | 27.9 |
| 3 | 25.2 | 25.5 | 28.0 |
���� ��1���ȸ��ݱ��вⶨ���ݼ�������Һ��Ӧǰ���ƽ���¶Ȳ�ٸ���Q=m•c•��T�������Ӧ�ų���������Ȼ����������1molˮ�ų����������Ϳ��Եõ��к��ȣ�
��2�������к��ȵIJⶨ���ܹ����·ų�������ƫ�͵����ؽ��н��
��3��Ϊ��ȷ��CH3COOH��Һ��ȫ���кͣ��Ӷ����ʵ���ȷ�ȣ�����NaOH�Թ�����
��4�����������ᣬ���������Ҫ����������
��� �⣺��1����1��ʵ��CH3COOH��NaOH��Һ��ʼƽ���¶�Ϊ25.05�棬��Ӧ���¶�Ϊ��27.8�棬�¶Ȳ�Ϊ��2.75�棻��2��ʵ��CH3COOH��NaOH��Һ��ʼƽ���¶�Ϊ25.2�棬��Ӧ���¶�Ϊ��27.9�棬�¶Ȳ�Ϊ��2.7�棻��3��ʵ��CH3COOH��NaOH��Һ��ʼƽ���¶�Ϊ25.35�棬��Ӧ���¶�Ϊ��28.0�棬�¶Ȳ�Ϊ��2.65�棻
�����¶Ȳ�ƽ��ֵΪ��2.7�棬100mL0.50mol•L CH3COOH��Һ ��100mL0.55mol•L-1NaOH��Һ��Ϸ�Ӧ������ˮ0.05mol���ų�������Q=cm��t=2.7K��4.184J•��g•K��-1��200g+150.5J•K-1��2.7K=2665.71J=2.67kJ����H=$\frac{-Q}{n��{H}_{2}O��}$=-$\frac{2.67kJ}{0.05mol}$=-53.3 kJ/mol��
�ʴ�Ϊ��-53.3 kJ/mol��
��2��CH3COOH���к��ȵ�����ֵΪ56.1KJ•mol-1��ʵ���ϲⶨ��ֵƫ�ͣ�����ԭ���У������ȼƵı���ƿЧ�����ã��������Һ��ϲ�Ѹ�٣����¶ȼƲ�����ȷ�ȣ�
�ʴ�Ϊ�������ȼƵı���ƿ����Ч�����ã��������Һ��ϲ�Ѹ�٣����¶ȼƲ�����ȷ�ȣ�
��3����ͼӦ���к���ʱ��Ϊ�˱�֤һ��ȫ����Ӧ��������Ҫ��һ�Լ����Թ���������ʵ��������ʵ����NaOH������Ϊ���ܱ�֤CH3COOH��Һ��ȫ���кͣ��Ӷ����ʵ���ȷ�ȣ�
�ʴ�Ϊ��ʹ�����Թ�����Ϊ���ܱ�֤CH3COOH��Һ��ȫ���кͣ��Ӷ����ʵ���ȷ�ȣ�
��4�����������ᣬ���������Ҫ��������������CH3COOH���к�����HCl���к�����ֵ��ȣ�HCl�ϴ�
�ʴ�Ϊ��HCl��CH3COOH�����ᣬֻ���ٲ��ֵ��룬CH3COOH��������ʱҪ���ȣ�
���� ���⿼���к��ȵIJⶨ��ע�����ղⶨ�к��ȵ�ԭ�������������Լ��������㹫ʽ��Ӧ�ã���Ŀ�ѶȲ���


 ֥�鿪���γ�������ϵ�д�
֥�鿪���γ�������ϵ�д� ����ѧ��ţ��Ӣ��ϵ�д�
����ѧ��ţ��Ӣ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ij��ȤС��ͬѧ���������װ�ã����мг�������������������ȴˮ��û�л��������ü��ȱ����ᡢŨH2SO4���Ҵ������ķ������Ʊ������������������鷴Ӧ�IJ��ָ�����ұ����������ķе�Ϊ213�棬���ѵķе�Ϊ34.6�森
ij��ȤС��ͬѧ���������װ�ã����мг�������������������ȴˮ��û�л��������ü��ȱ����ᡢŨH2SO4���Ҵ������ķ������Ʊ������������������鷴Ӧ�IJ��ָ�����ұ����������ķе�Ϊ213�棬���ѵķе�Ϊ34.6�森�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 2 0 1 2 0 2 | B�� | 0 2 1 0 1 2 | ||
| C�� | 2 4 3 2 2 6 | D�� | 2 10 6 2 5 12 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
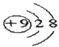 ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �٢ۢݢ� | B�� | �٢ڢܢ� | C�� | �٢ۢݢ� | D�� | �٢ۢޢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | Cl2+2FeCl2�T2FeCl3 | B�� | 3I2+6FeCl2�T4FeCl3+2FeI3 | ||
| C�� | Co2O3+6HCl�T2CoCl2+Cl2��+3H2O | D�� | 2Fe3++2I-�T2Fe2++I2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ���ݷ�Ӧ2C+SiO2$\frac{\underline{\;����\;}}{\;}$Si+2CO��˵��̼�ķǽ�����ǿ�ڹ�ķǽ����� | |
| B�� | ���ȷֽ�CuSO4•5H2O������ʹ�õIJ��������оƾ��ơ������������� | |
| C�� | ����ϼ�Ϊ+7��Ԫ��һ����������Ԫ�� | |
| D�� | 16gO3����8NA������ |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com