
| A�� | ��0.4mol/LNH4Cl��Һ��0.2molNaOH��Һ�������Ϻ���Һ�����ӵ����ʵ���Ũ�ȴ�С��ϵΪ��c��NH4+����c��Na+����c��NH3•H2O����c��OH-����c��H+�� | |
| B�� | SiO2��s��+2C��s���TSi��s��+2CO��g���ڳ����²����Է����У���÷�Ӧ�ġ�H��0 | |
| C�� | ���ʵ���Ũ����ȵ�CH3COOH��CH3COONa��Һ�������Ϻ����Һ�У�2c��Na+���Tc��CH3COO-��+c��CH3COOH�� | |
| D�� | ����ͬ�����£�NaHCO3��Һ��NaHSO3��Һ�У�ˮ�ĵ���ƽ������ٽ� |
���� A.0.4mol/LNH4Cl��Һ��0.2molNaOH��Һ�������Ϻ�����Ϊ������NH4Cl��NaCl��NH3��H2O���������ˮ�⣬�Լ��ԣ�
B���÷�Ӧ�ġ�S��0���ڳ����²����Է����У����H-T��S��0��
C��CH3COOH��CH3COONa��Һ�������ϣ����ʵ�����ͬ����ѭ�����غ㣻
D��NaHSO3��Һ�����ԣ��������ˮ�⣮
��� �⣺A.0.4mol/LNH4Cl��Һ��0.2molNaOH��Һ�������Ϻ�����Ϊ������NH4Cl��NaCl��NH3��H2O���������ˮ�⣬�Լ��ԣ�������Ũ��Ϊc��NH4+����c��Na+����c��NH3•H2O����c��OH-����c��H+������A��ȷ��
B���÷�Ӧ�ġ�S��0���ڳ����²����Է����У����H-T��S��0����֪�÷�Ӧ�ġ�H��0����B��ȷ��
C��CH3COOH��CH3COONa��Һ�������ϣ����ʵ�����ͬ����ѭ�����غ㣬��2c��Na+���Tc��CH3COO-��+c��CH3COOH������C��ȷ��
D��NaHSO3��Һ�����ԣ��������ˮ�⣬����ˮ�ĵ��룬��NaHCO3��Һ�ٽ�ˮ�ĵ���ƽ�⣬��D����
��ѡD��
���� ���⿼������Ũ�ȴ�С�ıȽϣ�Ϊ��Ƶ���㣬���ջ����Һ�����ʡ���Ӧ�����ʱ䡢�����غ㡢ˮ�������Ϊ���Ĺؼ������ط�����Ӧ�������Ŀ��飬ע��ѡ��DΪ�����״��㣬��Ŀ�ѶȲ���



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 0.5molCl2ͨ������ˮ�г�ַ�Ӧ��ת�Ƶĵ���������0.5NA | |
| B�� | 46gNO2��N2O4��������к���ԭ������Ϊ3NA | |
| C�� | ��״���£�22.4LSO3�к��еķ�����ΪNA�� | |
| D�� | 1L0.1mol/L��NaHSO3��Һ�У�HSO32-��SO32-��������֮��Ϊ0.1NA |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | CH3COO- | B�� | SO32- | C�� | CO32- | D�� | HCO3- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ԭ�Ӱ뾶��R��Q | B�� | R��Q���γ����ӻ�����R2Q5 | ||
| C�� | R��Q���γɹ��ۻ�����RQ | D�� | R��Q���γɹ��ۻ�����RQ2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
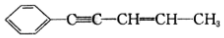 ���й���ṹ˵����ȷ���ǣ�������
���й���ṹ˵����ȷ���ǣ�������| A�� | ͬһƽ���ϵ�̼ԭ��������9�� | B�� | ����̼ԭ�ӿ�����ͬһ��ֱ���� | ||
| C�� | ����̼ԭ�Ӳ�������ͬһƽ���� | D�� | ����̼ԭ�ӿ�����ͬһƽ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
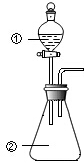 ��ͼ��ʾװ�ý�������ʵ�飺��������Һ������У�Ԥ���������ʵ��������ǣ�������
��ͼ��ʾװ�ý�������ʵ�飺��������Һ������У�Ԥ���������ʵ��������ǣ������� | ѡ�� | �������� | �������� | Ԥ����е����� |
| A | Ũ���� | MnO2 | ��������ɫ���� |
| B | Ũ���� | ��ɰֽ��ĥ�������� | ��������ɫ���� |
| C | �ữ��FeCl2��Һ | H2O2��Һ | ��Һ��ɻ���ɫ�������ݲ��� |
| D | Fe2��SO4��3��Һ | ͭ�� | ��Һ����ɫ���к�ɫ������� |
| A�� | A | B�� | B | C�� | C | D�� | D |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 1 mol•L-1��NaCl��Һ��ָ��1 mol NaCl��1 000 mLˮ���Ƴɵ���Һ | |
| B�� | ��1 L 0.5 mol•L-1��NaCl��Һ��ȡ��100 mL��ʣ����Һ���ʵ���Ũ��Ϊ0.45 mol•L-1 | |
| C�� | 0��ʱ��2 mol Cl2���������Ϊ22.4 L | |
| D�� | CaCl2��Ħ��������111 g |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com