
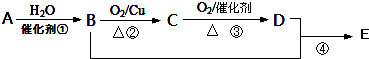
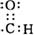 ��
������ A�IJ���ͨ����������һ�����ҵ�ʯ�ͻ���ˮƽ����AΪCH2=CH2����ˮ�����ӳɷ�Ӧ�õ�BΪCH3CH2OH��B�������õ�CΪCH3CHO��C�����õ�DΪCH3COOH���Ҵ������ᷢ��������Ӧ�õ�EΪ�����������ݴ˽��
��� �⣺A�IJ���ͨ����������һ�����ҵ�ʯ�ͻ���ˮƽ����AΪCH2=CH2����ˮ�����ӳɷ�Ӧ�õ�BΪCH3CH2OH��B�������õ�CΪCH3CHO��C�����õ�DΪCH3COOH���Ҵ������ᷢ��������Ӧ�õ�EΪ����������
��1��������������֪��B�Ľṹ��ʽΪCH3CH2OH��CΪCH3CHO��C ����������Ϊȩ���������ʽΪ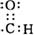 ��
��
�ʴ�Ϊ��CH3CH2OH��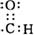 ��
��
��2��������������֪��B+D��E�ķ�Ӧ����Ϊ������Ӧ��
�ʴ�Ϊ��������Ӧ��
��3��CΪCH3CHO��C �й�����Ϊȩ��������ȩ�����Լ�Ϊ����������ͭ����Һ��������Һ��
�ʴ�Ϊ������������ͭ����Һ��������Һ��
��4��B��C�Ļ�ѧ��Ӧ����ʽΪ��2 CH3CH2OH+O2$��_{��}^{Cu}$2 CH3CHO+2H2O��B+D��E�Ļ�ѧ��Ӧ����ʽΪ��CH3COOH+CH3CH2OH$?_{��}^{ŨH_{2}SO_{4}}$CH3COOCH2CH3+H2O��
�ʴ�Ϊ��2 CH3CH2OH+O2$��_{��}^{Cu}$2 CH3CHO+2H2O��CH3COOH+CH3CH2OH$?_{��}^{ŨH_{2}SO_{4}}$CH3COOCH2CH3+H2O��
���� ���⿼�������ƶϣ��漰ϩ��������ȩ�������������ת�����Ƚϻ��������ضԻ���֪ʶ�Ĺ��̣�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| ��ѧʽ | ���볣�� |
| CH3COOH | Ka=1.76��10-5 |
| H2SO3 | ${K_{a_1}}$=1.54��10-2 |
| ${K_{a_2}}$=1.02��10-7 | |
| HF | Ka=6.03��10-4 |
| A�� | 1mol•L-1NaHA��Һ��һ�����ڣ�c��Na+��=c��H2A��+c��HA-��+c��A2-�� | |
| B�� | ���������Һ�м����������ᣬ�õ������Ի����Һ�У�c��Na+����c��CH3COO-����c��H+����c��OH-�� | |
| C�� | pH������3�Ĵ�����������Һ�������Ϻ���Һ��pH��� | |
| D�� | ��֪ij�¶��³�������ĵ���ƽ�ⳣ���������ͬ���ʵ���Ũ�ȵ�CH3COONa��NaF��Na2SO3��NaHSO3ˮ��Һ����Һ������������С�������е�˳����Na2SO3��CH3COONa��NaF��NaHSO3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| ʵ����� | ����ˮ����� | KI��Һ����� | ���ĵ�Na2S2O3��Һ����� |
| 1 | 10.00mL | 10.00mL | 19.96mL |
| 2 | 10.00mL | 10.00mL | 20.04mL |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
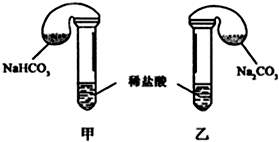
| �Լ����� | ʵ������ ����������仯�� | ����ԭ�� | |
| �� �� �� | 42g NaHCO3 53g Na2CO3 300mL4mol/L���� | �������������� ����������� | �ס������������ n��NaHCO3��=n ��Na2CO3�� V����CO2��=V����CO2�� |
| �� �� �� | 42g NaHCO3 53g Na2CO3 300mL3mol/L���� | ���������������������������������ڡ���С�ڡ� ���ڡ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
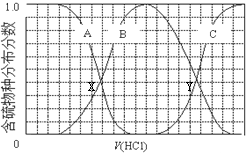
| A�� | ��������B��ʾHS- | |
| B�� | �ڵμ���������У���Һ��c��Na+���뺬�������Ũ�ȵĴ�С��ϵΪ��c��Na+��=3[c��H2S��+c��HS-��+c��S2-��] | |
| C�� | X��YΪ���ߵ�������㣬����֪��X�㴦��pH���Ϳ��Լ����H2S��Kaֵ | |
| D�� | NaHS�ʼ��ԣ�������Һ�м���CuSO4��Һ��ǡ����ȫ��Ӧ��������Һ��ǿ���ԣ���ԭ����Cu2++HS-�TCuS��+H+ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ԭ�Ӱ뾶A��B��D��C | B�� | ԭ������a��b��c��d | ||
| C�� | ���Ӱ뾶D��C��B��A | D�� | ������B��A���ǽ�����D��C |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| ʱ��/s | 0 | 2 | 4 | 6 | 8 | 10 |
| c��NO��/mol•L-1 | 1.00��10-3 | 4.50��10-4 | 2.50��10-4 | 1.50��10-4 | 1.00��10-4 | 1.00��10-4 |
| c��CO��/mol•L-1 | 3.60��10-3 | 3.05��10-3 | 2.85��10-3 | 2.75��10-3 | 2.70��10-3 | 2.70��10-3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
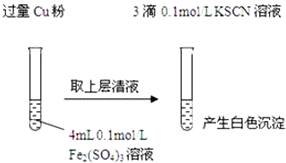
| ʵ�鷽�� | ���� | ���� |
| ����1��ȡ4mL0.1mol/L CuSO4��Һ�������еμ�3��0.1mol/L KSCN��Һ | ������ɫ���� | CuSO4��KSCN��Ӧ�����˰�ɫ���� |
| ����2��ȡȡ4mL0.1mol/LFeSO4��Һ�������еμ�3��0.1mol/LKSCN��Һ | ���������� |
| ʵ�鷽�� | ���� |
| ��3mL 0.1mol/L FeSO4��Һ�м���1mL 8mol/Lϡ���� | ��Һ��Ϊ��ɫ������һ��ʱ�����ɫ��ʧ����Һ��Ϊ��ɫ |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com