
���� ��1������ȼ���ȵĸ�����Ȼ�ѧ����ʽ����д����д����ע�����ʵľۼ�״̬���ʱ�ļ��㣻
��2�������Ȼ�ѧ����ʽ����д������֪�����ʵ����ʵ����뷴Ӧ�ų������������ȣ�0.2mol��̬����ȼ����������������ȼ�գ����ɹ�̬�����������Һ̬ˮ���ų�433.0kJ����������1mol��̬����ȼ����������������ȼ�գ����ɹ�̬�����������Һ̬ˮ���ų�433.0��5=2165.0kJ��������ע����������ʵľۼ�״̬�����
��3������д����Ӧ�Ļ�ѧ����ʽ����2CO2��g��+6H2��g��=CH3OCH3��g��+3H2O��g����Ȼ�����ø�˹���ɽ��⣮
��� �⣺��1��1g�������ʵ���Ϊ$\frac{1}{58}$mol����ȫȼ�ղ����ɶ�����̼��Һ̬ˮʱ���ų�����Ϊ50kJ������1mol������ȫȼ�������ȶ�������ʱ�ų�������Ϊ50kJ��58=2900kJ��ȼ������1mol��ȼ����ȫȼ�������ȶ�������ʱ�ų�����������ʾ����ȼ���ȵ��Ȼ�ѧ����ʽ��C4H10��g��+$\frac{13}{2}$O2��g���T4CO2��g��+5H2O��H=-2900kJ/mol��
�ʴ�Ϊ��C4H10��g��+$\frac{13}{2}$O2��g���T4CO2��g��+5H2O��H=-2900kJ/mol��
��2��0.2mol��̬����ȼ����������������ȼ�գ����ɹ�̬�����������Һ̬ˮ���ų�433.0kJ����������1mol��̬����ȼ����������������ȼ�գ����ɹ�̬�����������Һ̬ˮ���ų�433.0��5=2165.0kJ���������Ȼ�ѧ����ʽ�з��ȡ�H��0�����ԡ�H=-2165.0kJ/mol�����Ը÷�Ӧ���Ȼ�ѧ����ʽΪB2H6��g��+3O2��g���TB2O3��s��+3H2O��l����H=-2165.0kJ/mol��
�ʴ�Ϊ��B2H6��g��+3O2��g���TB2O3��s��+3H2O��l����H=-2165.0kJ/mol��
��3�����ø�˹���ɽ��м��㣬����������ʽ������ʽ�任��CO��g��+2H2��g���TCH3OH��g����H=-90.7kJ•mol-1
����2�ã�2CO��g��+4H2��g��=2CH3OH��g����H=-181.4 kJ•mol-1��
CO��g��+H2O��g���TCO2��g��+H2��g����H=-41.2kJ•mol-1������-2�ã�2CO2��g��+2H2��g��=2CO��g��+2H2O��g����H=+82.4 kJ•mol-1��
��2CO��g��+4H2��g��=2CH3OH��g����H=-181.4 kJ•mol-1
��2CH3OH��g=CH3OCH3��g��+H2O��g����H=-23.5 kJ•mol-1
��2CO2��g��+2H2��g��=2CO��g��+2H2O��g����H=+82.4 kJ•mol-1
�٢ڢ���ʽ��ӵã�2CO2��g��+6H2��g��=CH3OCH3��g��+3H2O��g����H=-122.5 kJ•mol-1
�ʴ𰸣�2CO2��g��+6H2��g��=CH3OCH3��g��+3H2O��g����H=-122.5 kJ•mol-1��
���� ������Ҫ�������Ȼ�ѧ����ʽ����д����Ҫע����У����ʵ�״̬����Ӧ�ȵ���ֵ�뵥λ����Ӧ�ȵ���ֵ�뻯ѧ����ʽǰ���ϵ�������ȣ�ע���˹���ɵ����ã�ȼ���ȵĸ����Ŀ�Ѷ��еȣ�


 ��ǰ����ϵ�д�
��ǰ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��AgNO3������AgCl��AgI���ɳ���������AgClΪ�� | |
| B�� | ����Һ��ϣ�AgCl��AgI������ | |
| C�� | ��ȡ0.1435 g AgCl�������100mLˮ����������仯����c��Cl-��Ϊ0.01 mol/L | |
| D�� | ��AgI��Һ����AgNO3��c��Ag+������Ksp��AgI��Ҳ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
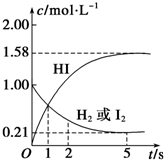 ij�¶�ʱ����ijV L���ܱ������г���3molH2��g����3mol I2��g����������Ӧ��
ij�¶�ʱ����ijV L���ܱ������г���3molH2��g����3mol I2��g����������Ӧ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| ��ѧ�� | H-H | N-H | N��N |
| ����kJ/mol | 436 | 391 | 945 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
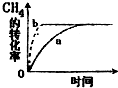 ̼���仯���������������������е�Ӧ�÷dz��㷺������̼�������ֻ��һ�����룬����һ��ֵ���ڴ������ʽ��
̼���仯���������������������е�Ӧ�÷dz��㷺������̼�������ֻ��һ�����룬����һ��ֵ���ڴ������ʽ��| ʵ���� | �¶�/�� | ��ʼ��/mol | ƽ����/mol | �ﵽƽ������ʱ��/min | ||
| H2O | CO | CO2 | CO | |||
| 1 | 650 | 2 | 4 | 1.6 | 2.4 | 5 |
| 2 | 900 | 1 | 2 | 0.4 | 1.6 | 3 |
| 3 | 900 | a | b | c | D | t |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
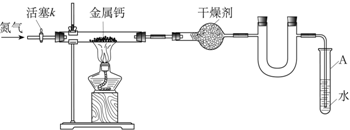
| ��Ӳ����������m0/g | Ӳ��������Ƶ�����m1/g | Ӳ����������������m2/g |
| 114.8 | 120.8 | 122.2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| ʵ���� | NaOH��ʼ���� | NaOH�յ���� |
| ��һ�� | 0.10mL | 18.40mL |
| �ڶ��� | 3.00mL | 21.10mL |
| ������ | 0.20mL | 20.40mL |
| ���Ĵ� | 0.00mL | 18.20mL |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �ڲⶨ�к��ȵ�ʵ���У�Ϊ��ȷ�ⶨ��Ӧ�����Һ���¶ȣ�ʵ�����¶ȼ�ˮ����Ӧ��С�ձ��ײ��Ӵ� | |
| B�� | ����к͵ζ�ʵ���У���ƿҪ�ô�װҺԤ����ϴ | |
| C�� | �ڲⶨ�к��ȵ�ʵ���У���0.5mol•L-1NaOH��Һ�ֱ���0.5 mol•L-1�����ᡢ������Һ��Ӧ������ȡ����Һ�����ȣ����õ��к�����ֵ��ͬ | |
| D�� | ����ҺPHֵʱ��PH��ֽ������ʪ |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com