
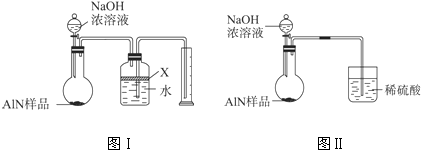
| aL |
| 22.4L/mol |
| a |
| 22.4 |
| a |
| 22.4 |
| a |
| 22.4 |
| 41a |
| 22.4 |
| ||
| wg |
| 4100a |
| 22.4w |
| 4100a |
| 22.4w |


 Źī¼Ł×÷ŅµŹī¼ŁæģĄÖĮ·Ī÷°²³ö°ęÉēĻµĮŠ“š°ø
Źī¼Ł×÷ŅµŹī¼ŁæģĄÖĮ·Ī÷°²³ö°ęÉēĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
| A£®¢Ł¢Ū¢Ü¢Ž¢į¢ā | B£®¢Ł¢Ü¢ß¢ą¢į¢ā | C£®¢Ł¢Ū¢Ü¢ß¢ą¢į | D£®¢Ł¢Ś¢Ü¢Ż¢ą¢į |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĪŹ“šĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĪŹ“šĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĪŹ“šĢā
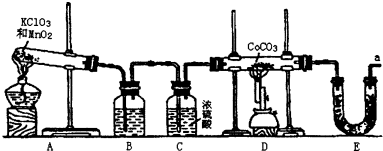
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā
| µĪ¶Ø“ĪŹż | “ż²āČÜŅŗµÄĢå»ż/mL | ±ź×¼ČÜŅŗµÄĢå»ż | |
| µĪ¶ØĒ°æĢ¶Č/mL | µĪ¶ØŗóæĢ¶Č/mL | ||
| 1 | 25.00 | 1.05 | 21.04 |
| 2 | 25.00 | 1.50 | 24.50 |
| 3 | 25.00 | 0.20 | 20.21 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĪŹ“šĢā
| ŹµŃé²Ł×÷ | Ō¤ĘŚĻÖĻóÓė½įĀŪ | ||
| ²½Öč1£ŗȔɣĮæ“ż²āŅŗ·ÅČėŹŌ¹Ü1ÖŠ£¬µĪ¼Ó¹żĮælmol/LĀČ»Æ±µČÜŅŗ£® | Čō²»³öĻÖ»ė×Ē£¬ŌņČÜŅŗÖŠ²»“ęŌŚSO32-£¬ Čō³öĻÖ»ė×Ē£¬ŌņČÜŅŗÖŠæÉÄÜŗ¬ÓŠSO32-£® | ||
| ²½Öč2£ŗČō³öĻÖ»ė×Ē£¬¾²ÖĆŅ»¶ĪŹ±¼äŗ󣬽«ÉĻ²ćĒåŅŗµ¹ČėŹŌ¹Ü2ÖŠ£®ĶłŹŌ¹Ü1ÖŠ¼ÓČėÕōĮóĖ®Ļ“µÓ³Įµķ£¬¾²ÖĆŗóʜȄÉĻ²ćĒåŅŗ£¬ŌŁ¼ÓČė______£® | ______ | ||
| ²½Öč3£ŗ______ |
|
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗŹµŃéĢā

²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com