��2010?��Զģ�⣩Fridel-Crafts��Ӧ�����������������Ҫ�ķ������ںϳ����кܴ��ʵ�ü�ֵ���÷�Ӧ���Լ�ʾ���£�ArH+RX
ArR+HX����H��0��Ar��ʾ��������ij��ѧ��ȤС����ʵ�����������嶡�������ᷴӦ�Ƶ��嶡���ȣ��е�50.7�棩��������Fridel-Crafts��Ӧԭ���Ʊ����嶡�����ӣ��۵�99�棩����Ӧ���̼�ʵ��װ������ͼ��ʾ��
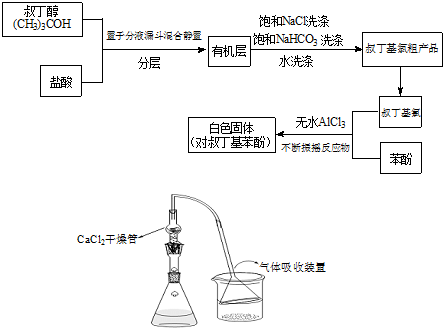
�Իش��������⣺
��1����д����ʵ������е�������Ҫ��ѧ��Ӧ����ʽ��
��CH3��3COH+HCl����CH3��3CCl+H2O
��CH3��3COH+HCl����CH3��3CCl+H2O
��

+��CH
3��
3CCl
+HCl

+��CH
3��
3CCl

+HCl
��
��2���л����м��뱥��ʳ��ˮ������̼�����Ƶ����ÿ����ǣ�
��ȥ�嶡���ȴֲ����е�HCl
��ȥ�嶡���ȴֲ����е�HCl
��ͨ��
����
����
�����ɽ��嶡���ȴֲ���ת��Ϊ��Ϊ�������嶡���ȣ�
��3����ȥ�����Ʊ�װ���е��Ȼ��Ƹ���ܣ��п��ܵ��µIJ�������ǣ��û�ѧ����ʽ���������˵������
AlCl3+3H2O=Al��OH��3+3HCl�����������Ȼ���ˮ�����
AlCl3+3H2O=Al��OH��3+3HCl�����������Ȼ���ˮ�����
��
��4���嶡�����뱽�ӷ�Ӧʱ�ʵ������¶��Ǻ���Ҫ�ģ�����Ӧ�������¶ȹ���Ӧ����ˮԡ��ȴ��������ܵ��µIJ�������ǣ�
��Ӧ����ʹ�¶����ߣ������ɴ�����HCl���壬������ʱ��ȴ���嶡���ȵ��������ݳ���Ӱ�����
��Ӧ����ʹ�¶����ߣ������ɴ�����HCl���壬������ʱ��ȴ���嶡���ȵ��������ݳ���Ӱ�����
��
��5���������嶡�������ڷ�����ȥ��Ӧ��ʵ�������õı����������䣬��д���嶡���ȷ�����ȥ��Ӧ�Ļ�ѧ����ʽ��
��CH3��3CCl��CH2=C��CH3��2+HCl��
��CH3��3CCl��CH2=C��CH3��2+HCl��
��
��6����ʱ�����ղ�Ʒ���嶡�����Ӳ��ǰ�ɫ��������ɫ������Ϊ���ܵ�ԭ���ǣ�
һ���ֱ��ӱ������е�����������
һ���ֱ��ӱ������е�����������
��


 +��CH3��3CCl
+��CH3��3CCl +HCl
+HCl +��CH3��3CCl
+��CH3��3CCl +HCl
+HCl +��CH3��3CCl
+��CH3��3CCl  +HCl��
+HCl�� +��CH3��3CCl
+��CH3��3CCl  +HCl��
+HCl��


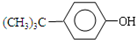
 ��2010?��Զģ�⣩�Ե������Ǵӷ仨��ֲ������ȡ�����������ʣ���ṹ��ͼ��ʾ������������ȷ���ǣ�������
��2010?��Զģ�⣩�Ե������Ǵӷ仨��ֲ������ȡ�����������ʣ���ṹ��ͼ��ʾ������������ȷ���ǣ�������