
��ͼΪʵ����ijŨ�����Լ�ƿ�ϵı�ǩ���Ը����й����ݻش��������⣺
��1����Ũ��������ʵ���Ũ��Ϊ
12.0
12.0
mol/L��
��2��ȡ����������ĸ�������Һʱ�������������в�����ȡ����Ķ��ٶ��仯����
BD
BD
��
A����Һ��HCl�����ʵ��� B����Һ��Ũ��
C����Һ��Cl
-����Ŀ D����Һ���ܶ�
��3��ijѧ����������Ũ���������ˮ����450mL���ʵ���Ũ��Ϊ0.3mol/Lϡ���ᣮ
����Ҫʹ�õ���Ҫ��������Ͳ���ձ�����������
500ml����ƿ
500ml����ƿ
��
��ͷ�ι�
��ͷ�ι�
��
�����������ɷֽ�Ϊ���¼�����
A������Ͳ��ȡ
12.5
12.5
mLŨ���ᣬ����ע��װ��Լ50mL����ˮ���ձ�����ò��������裮
B������������ˮ������ϴ���ձ��Ͳ���������ÿ�ε�ϴҺ����������ƿ�
C����ϡ�ͺ������С�ĵ��ò���������������ƿ�
D���������ƿ�Ƿ�©ˮ��
E��������ˮֱ�Ӽ�������ƿ����Һ��ӽ��̶���1-2cm����
F���ǽ�ƿ���������ߵ���ҡ����Һ��
G���ý�ͷ�ι�������ƿ����μ�������ˮ����Һ����͵�ǡ����������У�
������������еĿհ״���
�۲��������ȷ�IJ���˳������ĸ��д����
�� D ������ A ������ C ����
B
B
��
E
E
��
G
G
���� F ����
��4�������ƹ����У�����ʵ������������Ƶ�ϡ��������ʵ���Ũ���к�Ӱ�죿
�����������ƫ�ߡ���ƫ�͡�����Ӱ�족����
������Ͳ��ȡŨ����ʱ���ӹ۲찼Һ��
ƫ��
ƫ��
�ڶ���ʱ���۾����ӿ̶��ߣ�
ƫ��
ƫ��
�۶��ݺӸǵ�תҡ�Ⱥ���������ڿ̶��ߣ��ֵμ�����ˮ���̶ȣ�
ƫ��
ƫ��
��

 ��ͼΪʵ����ijŨ�����Լ�ƿ��ǩ�ϵ��й����ݣ��Ը��ݱ�ǩ�ϵ��й����ݻش��������⣺
��ͼΪʵ����ijŨ�����Լ�ƿ��ǩ�ϵ��й����ݣ��Ը��ݱ�ǩ�ϵ��й����ݻش��������⣺

 ��У������Ԫͬ��ѵ��������ϵ�д�
��У������Ԫͬ��ѵ��������ϵ�д�
 ��ͼΪʵ����ijŨ�����Լ�ƿ�ϵı�ǩ���Ը����й����ݻش��������⣺
��ͼΪʵ����ijŨ�����Լ�ƿ�ϵı�ǩ���Ը����й����ݻش��������⣺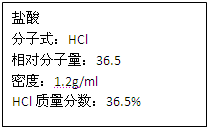 ��ͼΪʵ����ijŨ�����Լ�ƿ�ϵı�ǩ���Ը����й����ݻش��������⣺
��ͼΪʵ����ijŨ�����Լ�ƿ�ϵı�ǩ���Ը����й����ݻش��������⣺
 ��ͼΪʵ����ijŨ�����Լ�ƿ�ı�ǩ�ϵ��й����ݣ��Իش��������⣺
��ͼΪʵ����ijŨ�����Լ�ƿ�ı�ǩ�ϵ��й����ݣ��Իش��������⣺ ��ͼΪʵ����ijŨ�����Լ�ƿ�ϵı�ǩ���й����ݣ��Ը��ݱ�ǩ�ϵ��й����ݻش��������⣺
��ͼΪʵ����ijŨ�����Լ�ƿ�ϵı�ǩ���й����ݣ��Ը��ݱ�ǩ�ϵ��й����ݻش��������⣺