
���� ��1�����ݵ����غ��ԭ���غ�����ƽ��ѧ����ʽ��
��2��������ԭ��Ӧ�У����ϼ�����Ԫ��ʧȥ���ӣ����ϼ۽���Ԫ�ض�Ӧ�IJ����ǻ�ԭ������ݻ�ѧ����ʽ�����㷴Ӧת�Ƶ��ӵ�����
��3�����ݻ�ѧ����ʽ����������Ӻͽ�����֮��Ĺ�ϵ������ش�
��4������Һ��Al2O3��Ӧ����Al3+���ɣ�˵������Һ�����ԣ����������ܷ�Ӧ�����Ӳ��ܴ������棻
�ڸ���Һ��Al2O3��Ӧ����AlO2-����˵������Һ�Լ��ԣ��������������ӷ�Ӧ�����ӿɴ������棻
�ۼ�����������Ӧ����ƫ��������Ӻ�ˮ��
��� �⣺��1����Ԫ�ػ��ϼ۹�������3�ۣ�����Ԫ�ػ��ϼ۹�������10�ۣ����ݵ�ʧ�����غ㣬����Ҫת�Ƶ���30mol�����Խ�������ǰ��ϵ����10�������ǰ��ϵ����6������ǰ��ϵ����3������ԭ���غ���ƽ��ԭ�Ӻ���ԭ�Ӽ��ɣ���
6NO3-+10Al+18H2O�T3N2��+10Al��OH��3+6OH-���ʴ�Ϊ��6��10��18��3��10��6��
��2��������ԭ��Ӧ�У����ϼ�����Ԫ��Alʧȥ���ӣ����ϼ۽��͵�NԪ�ض�Ӧ�IJ��ﵪ���ǻ�ԭ������ݻ�ѧ����ʽ����֪��������3mol�ĵ���ת�Ƶ�����30mol��������1mol����ʱ��ת�Ƶ�����10mol��
�ʴ�Ϊ��Al��10mol��
��3����Ϊ����0.3mol�ķ�ˮ�е�NO3-�����ʵ���Ϊ0.3mol�����ݻ�ѧ����ʽ��0.3mol��������������Ľ����������ʵ���Ϊ0.5mol��
�ʴ�Ϊ��0.5 mol��
��4������Һ��Al2O3��Ӧ����Al3+���ɣ�˵������Һ�����ԣ�һ������H+��OH-��HCO3-һ������������ڣ�
�ʴ�Ϊ��H+��OH-��HCO3-��
�ڸ���Һ��Al2O3��Ӧ����AlO2-����˵������Һ�Լ��ԣ�H+��Mg2+��Ag+��OH-���ܴ������棬�ض�����һ�������ӣ�Na+������Һ��һ������OH-��Na+�����ܺ���Cl-��NO3-��
�ʴ�Ϊ��OH-��Na+��Cl-��NO3-��
�ۼ�����������Ӧ����ƫ��������Ӻ�ˮ�������ӷ�ӦΪAl2O3+2OH-�T2AlO2-+H2O��
�ʴ�Ϊ��Al2O3+2OH-�T2AlO2-+H2O��
���� ���⿼����������ԭ��Ӧ�����ӵĹ��棬Ϊ�߿��������ͣ�ע����Ϣ�ij�ȡ��������ԭ��Ӧ�Ŀ��飬��ѧ��˼ά�������нϺõ�ѵ�������м�ʵ�Ļ���֪ʶ���ɽ��ע�������������ԣ���Ŀ�Ѷ��еȣ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��ͬ���ʵ���Ũ�ȵ�FeI2��Һ����ˮ�������� 2Fe2++2I-+2Br2�T2Fe3++I2+4Br- | |
| B�� | Ba��OH��2��Һ�м��������NaHSO4��Һ Ba2++OH-+H++SO42-�TH2O+BaSO4�� | |
| C�� | ������������Һ��ϡ������ Fe��OH��2+2H+�TFe2++2H2O | |
| D�� | ��Ư����Һ��ͨ�������Ķ�����̼ Ca2++2ClO-+CO2+H2O�TCaCO3��+2HClO |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �Ȼ�����Һ�ڵ��������µ����K+��Cl- | |
| B�� | ������ԭ��Ӧ�ı������л��ϼ۵����� | |
| C�� | ���ݶ����ЧӦ�ɽ���ɢϵ��Ϊ��Һ����������Һ | |
| D�� | ��������������ڽ��������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
| A�� | ����ʯ�����CO32-+2H+�TH2O+CO2�� | |
| B�� | ����ϡ���2Fe+6H+�T2Fe3++3H2�� | |
| C�� | ϡ����Ͱ�ˮ��H++NH3•H2O�TNH4++H2O | |
| D�� | ̼�������Һ�����HCO3-+H+�TH2O+CO2�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
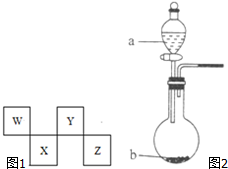 W��X��Y��Z���ֶ�����Ԫ�������ڱ��е�λ����ͼ1��ʾ������Y����Ԫ�غ���Ԫ�ؾ����γ�ԭ�Ӹ���1��1��1��2�Ļ����
W��X��Y��Z���ֶ�����Ԫ�������ڱ��е�λ����ͼ1��ʾ������Y����Ԫ�غ���Ԫ�ؾ����γ�ԭ�Ӹ���1��1��1��2�Ļ���� ��Z�����ڱ��е�λ�õ������ڢ�A�壮
��Z�����ڱ��е�λ�õ������ڢ�A�壮�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 88.0g �ɱ��к��еĵ�����Ϊ8.0NA | |
| B�� | �����£�11.2L��ϩ����������ȫȼ��ת�Ƶĵ�����Ϊ6.0NA | |
| C�� | ������1.0L0.1 mol•L-1NH4Cl ��Һ�У�NH4+��H+��������0.1NA | |
| D�� | 1.2g���ʯ�к��е�̼̼����Ϊ0.4NA |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | Fe���Ժ�S�ڼ�������������Fe2S3 | |
| B�� | Cl2��H2S�����ɷ�����Ӧ��H2S+Cl2�T2HCl+S�� | |
| C�� | ��������Һ�У�Fe3+��S2-���Դ������� | |
| D�� | Cl2��������Fe3+ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
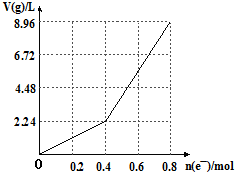
| A�� | ���ǰCuSO4��Һ�����ʵ���Ũ��Ϊ2mol/L | |
| B�� | ����������Һ��c��H+���T2mol/L | |
| C�� | ��n��e-��=0.6molʱ��V��H2����V��O2��=3��2 | |
| D�� | ��������Һ�м���16gCuO������Һ�ɻָ�Ϊ���ǰ��Ũ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 �ش��������⣺
�ش��������⣺�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com