
10��)����98%��Ũ���ᣨ�ѣ�1.84g/cm3�����Ƴ�Ũ��Ϊ0.5mol/L��ϡ����500mL��
��1��ѡ�õ���Ҫ�����У���Ͳ���ձ�������������ͷ�ιܣ�________��
��2�������������Ʋ�����
A����Ͳ��ȡŨH2SO4 B�����ߵ�ҡ�� C�ý�ͷ�ιܼ�ˮ���̶�
Dϴ���������������� E��ϡ��ŨH2SO4 F������Һת������ƿ
����ȷ�IJ���˳��Ϊ��___________ ��
��3����Ҫ�ش��������⣺
������Ũ��������Ϊ_______mL��
�����ʵ������15mL ��20mL�� 50mL����Ͳ��Ӧѡ�� _____mL����Ͳ��ã���ȡʱ������Ͳ���ɾ�����ˮϴ����ֱ����ȡ��ʹ���Ƶ�Ũ��_____________��ƫ�ߡ�ƫ�͡���Ӱ�죩��
10��)��1�� 500 m L����ƿ����2��D A E F C B ��3�� 13.6 15, ƫ��
���������������1����Һ������500mL��Һʱ���õ���������Ͳ���ձ�������������ͷ�ι��⺣��500mL������ƿ��
��2��������Һ����ϴ��������Ȼ����ȡ��Һ��������ܽ⣨��ϡ�ͣ�����Һ��ϴ�Ӻ�����Һ�����ݣ�ҡ�ȣ�������ȷ�IJ���˳��Ϊ��D A E F C B��
��3����98%��Ũ���ᣨ�ѣ�1.84g/cm3�������ʵ���Ũ����1000��1.84��98%/98=18.4mol/L��������Ũ����������xL����18.4x=0.5mol/L��0.5L��x=0.0136L��������ҪŨ����������13.6ml��
��ѡ����Ͳʱ������ȡҺ������ȷ��������ԽСԽȷ������ѡ��15mL����Ͳ�Ϻã���ȡʱ������Ͳ���ɾ�����ˮϴ����ֱ����ȡ���൱��ϡ����Ũ���ᣬ��ʹ���Ƶ�Ũ��ƫ�͡�
���㣺������Һ�����Ʋ��������ķ���



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
Ϊ���ȫ����Դ�뻷�����⣬���ܼ����ѳɹ�ʶ�����д�ʩ�����ڽ��ܼ��ŵ���
| A�����С�����һСʱ��Ϩ�ƻ |
| B��¶����յ��ݺͽո� |
| C�����콫�յ����¶�������26������ |
| D����������������մ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
��14�֣�ijͬѧ����ˮ�ʼ��վ����800mL 1 mol��L��1NaOH��Һ�Ա�ʹ�á�
��1����ͬѧӦѡ��IJ������������ձ�����Ͳ������������ͷ�ι��⣬����___________��
��2���������������ͼ��ʾ������ͼ����Ӧ����ͼ�е� (��ѡ����ĸ)֮�䡣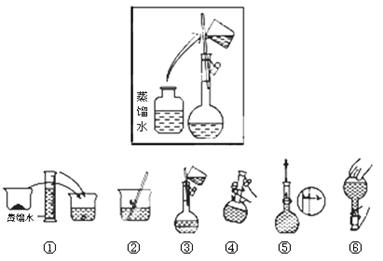
A������� B������� C�������
��3����ͬѧӦ��������ƽ��ȡNaOH���� g��������Ϊ33��1 g���ձ�����������ƽ�ϳ�ȡ����NaOH����ʱ��������ͼ��ѡ������ȷ��ʾ����λ�õ�ѡ�� ����ѡ����ĸ����
��4�����в�����������Һ��Ũ�ȴ�С�к�Ӱ�� (�ƫ����ƫС������Ӱ�족)��
�ٶ���ʱ�����Ӷ�����Ũ�Ȼ� ��
��ת����Һ�����У�����Һ�彦������Ũ�Ȼ� ��
������ƿδ���Ũ�Ȼ� ��
�ܶ���ҡ�Ⱥ�����Һ������ڿ̶��ߣ�Ũ�Ȼ� ��
��5��������Һ��ʵ�ʲ��������У�����Ҫ�죬�������� ����ʹ���Ƶ�NaOH��Һ��Ũ�ȱ�1 mol��L��1 �����С������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ʵ������Ҫ0.5 mol��L��1������Һ480 ml��������������Һ����������ش��������⣺
��1����ͼ��ʾ��������������Һ��Ҫ����______(�����)����ʹ������B��C��������ǰ��Ӧ���еIJ��� ��
��2�����в����У�����ƿ�����߱��Ĺ�����______ __(�����)��
A������һ�����ȷŨ�ȵı���Һ
B��������Һ
C����������ƿ������µ����������Һ��
D��ȷϡ��ijһŨ�ȵ���Һ
��3�����ݼ���,����Ͳ��ȡ18.4 mol��L��1��Ũ�������Ϊ___ ___ ml, ���ʵ������10 mL��15 mL��20 mL��Ͳ��ѡ�� ml����Ͳ��á���ʵ����������������ȷ��������ʱ���ӿ̶��ߣ���������ҺŨ��________0.5 mol��L��1(����ڡ��������ڡ���С�ڡ�����ͬ)����Ũ�������ձ����ܽ��δ��ȴ�����¾�ת��������ƿ����������ҺŨ��________0.5 mol��L��1��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
��ÿ��1�֣���8�֣�ʵ������Ҫ����0.1 mol��L��1CuSO4��Һ480 mL��
�����в������������ʵ������֣���ʹ��������������
��1��ѡ����������ɱ�ʵ��������������У�������ƽ(��ȷ��0.1 g)��ҩ�ס��ձ�����������______��________�Լ�����������Ƭ��ֽ��
��2�����㣬Ӧѡ��������ȷ________
| A����ҪCuSO4 ����8g | B����ҪCuSO4��5H2O����12.0 g |
| C����ҪCuSO4��5H2O����12.5 g | D����ҪCuSO4����7.7 g |

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
��10�֣���84����Һ������Чɱ�����H1N1������ijͬѧ������һƿ����¶ʿ���ơ�84����Һ����������������Ϻ�����Һ��װ˵���õ�������Ϣ����84����Һ������25%NaClO��1 000 mL���ܶ�1.192 g��cm��3��ϡ��100��(�����)��ʹ�á�
�����������Ϣ�����֪ʶ�ش��������⣺
��1���á�84����Һ�������ʵ���Ũ��Ϊ________mol��L��1��
��2����ͬѧȡ100 mL����¶ʿ���ơ�84����Һ��ϡ�ͺ�����������ϡ�ͺ����Һ��
c(Na��)��________mol��L��1��
��3��һƿ����¶ʿ���ơ�84����Һ�������տ�����________L��CO2(��״��)�����ʡ�(��֪��CO2��2NaClO��H2O===Na2CO3��2HClO)
��4����ͬѧ���ġ���¶ʿ���ơ�84����Һ�����䷽������NaClO��������480 mL��25%NaClO������Һ������˵����ȷ����________��
| A������ͼ��ʾ�������У��������Dz���Ҫ�ģ�����һ�ֲ������� |
| B������ƿ������ˮϴ����Ӧ��ɲ���������Һ���� |
| C�����ù������ƷNaClO�����ƿ��ܵ��½��ƫ�� |
| D����Ҫ������NaClO��������Ϊ143 g |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
����˵����ȷ����
| A��������ˮ����ͨ�����ȵ����ۣ����۱��ɫ |
| B����Fe(OH)3�����еμ�ϡH2SO4���ȳ��ֳ�����������ܽ� |
| C��������ʳ������ˮ���μӵ�����Һ������ɫ��˵�����Ǽӵ��� |
| D��Cu�Ľ��������Ա�Fe������ˮ����բ���Ͻ�װͭ��ɼ�������ʴ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
���������в����û��Ϸ�Ӧ�ķ����Ƶõ���
��SiO2 ��H2SiO3 ��Fe(OH)3 ��Al(OH)3 ��FeCl2 �� CaSiO3
| A���٢� | B���ڢ� | C���ڢۢܢ� | D���ڢܢ� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com