
| ���� | a | b | c | d | e |
| �е�(��) | 58.8 | 882.9 | 444.7 | 2 355 | 1 107 |
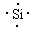 �� ԭ�ӣ� Si+2OH-+H2O��SiO32-+2H2��
�� ԭ�ӣ� Si+2OH-+H2O��SiO32-+2H2��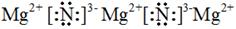
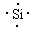 �����������γɵľ�����ԭ�Ӿ��塣���ʹ��ܺ�����������Һ��Ӧ����Ӧ�����ӷ���ʽ��Si+2OH-+H2O��SiO32-+2H2����
�����������γɵľ�����ԭ�Ӿ��塣���ʹ��ܺ�����������Һ��Ӧ����Ӧ�����ӷ���ʽ��Si+2OH-+H2O��SiO32-+2H2����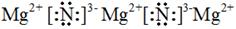 ��
��

 ����ѧ��ţ��Ӣ��ϵ�д�
����ѧ��ţ��Ӣ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
| ����ʽ | �ṹ��ʽ | ��� | �۵� | �ܽ��� |
| C12H10ClN3O | 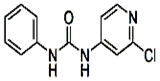 | ��ɫ�ᾧ��ĩ | 170��172�� | ������ˮ |
| | X | Y | Z |
| ��һ������(kJ/mol) | 520.2 | 495.8 | 418.8 |
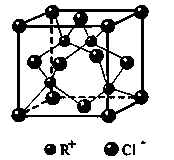
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�������
 �����Ȼ���Ļ�ѧʽΪ__________��
�����Ȼ���Ļ�ѧʽΪ__________���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
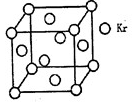
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ��ƶ���
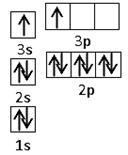
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��O2��O3��Ϊͬλ�� | B��O2ת��ΪO3Ϊ��ѧ�仯 |
| C����ͬ���ʵ�����O2��O3�������ͬ | D����ͬ������O2��O3������ͬ�ķ����� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| ѡ�� | ʵ����ʵ | ���۽��� |
| A | ��ԭ�ӵĵ�һ�����ܴ�����ԭ�� | ��ԭ��2p�ܼ������ |
| B | CO2Ϊֱ���η��� | CO2����Ϊ�Ǽ��Է��� |
| C | ���ʯ���۵����ʯī | ʯī����ʱ�����ƻ����ۼ��������ƻ����»��� |
| D | HF�ķе����HCl | H-F�ļ��ܴ���H-Cl |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
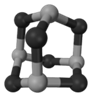
| A�����Ӽ� | B����� | C����λ�� | D�������� E.���Լ� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com