
����Ŀ�������仯�����������ҵ������������ҪӦ�á���ش��������⣺
(1)��ͼ��N2(g)��H2(g)��NH3(g)֮��ת����������ϵͼ����
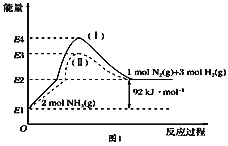
��N2(g)��H2(g)��Ӧ����NH3(g)���Ȼ�ѧ����ʽΪ___________________.
�ڹ���(��)����(��)�ķ�Ӧ��________(���ͬ����ͬ��).
��ij�¶��£���1 L���º��������г���1molN2��3 mol H2����������Ӧ��10 min�ﵽƽ�⣬��ʱ������ѹǿ��Ϊԭ����7/8.
a.�ù��̵�ƽ�ⳣ���ı���ʽΪ____________.
b.N2��ƽ��ת����Ϊ________.
c.��ʱ�����������¶Ⱥ�������䣬�������ټ���2.25 molN2��0.5 mol NH3����ƽ��________(�����������)�ƶ�.
(2)��NH3�������������������Ⱦ����֪��
��Ӧ��4NH3(g)��3O2(g)![]() 2N2(g)��6H2O(g) ��H1��a kJ��mol��1 ƽ�ⳣ��ΪK1
2N2(g)��6H2O(g) ��H1��a kJ��mol��1 ƽ�ⳣ��ΪK1
��Ӧ��N2(g)��O2(g)![]() 2NO(g) ��H2��b kJ��mol��1 ƽ�ⳣ��ΪK2
2NO(g) ��H2��b kJ��mol��1 ƽ�ⳣ��ΪK2
��Ӧ��4NH3(g)��6NO(g)![]() 5N2(g)��6H2O(g) ��H3��c kJ��mol��1 ƽ�ⳣ��ΪK3
5N2(g)��6H2O(g) ��H3��c kJ��mol��1 ƽ�ⳣ��ΪK3
��Ӧ���е�b��_____(�ú�a��c�Ĵ���ʽ��ʾ)��K3=_____(��K1��K2��ʾ).��Ӧ���еĦ�S______(�>����<������)0.
(3)�ں��ݵ��ܱ����У�����һ������NH3��NO����������Ӧ��ò�ͬ�¶��·�Ӧ��ϵ��NH3��ת����(��)��ѹǿp�Ĺ�ϵ��ͼ��ʾ��
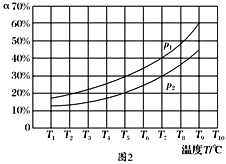
�ٷ�����p1________p2.(�>����<������)
�����������У�������Ϊ�жϷ�Ӧ���Ѿ��ﵽƽ��״̬�ı�־����________(�����).
a��N2��Ũ�Ȳ��ٸı� b������6 mol N��H����ͬʱ����6 mol H��O���γ�
c��������ѹǿ���ٱ仯 d�����������ܶȱ��ֲ���
���𰸡�N2(g)��3H2(g)![]() 2NH3(g) ��H����92 kJ��mol��1 ��ͬ K=c2(NH3)/��c(N2)c3(H2)�� 25% �� (a-c)/3
2NH3(g) ��H����92 kJ��mol��1 ��ͬ K=c2(NH3)/��c(N2)c3(H2)�� 25% �� (a-c)/3 ![]() > < bd
> < bd
��������
��1���پ�ͼ��֪2molNH3�ֽ�õ�1molN2��3molH2������92kJ/mol�����������N2(g)��H2(g)��Ӧ����NH3(g)���Ȼ�ѧ����ʽΪN2(g)��3H2(g) ![]() 2NH3(g) ��H����92 kJ��mol��1��
2NH3(g) ��H����92 kJ��mol��1��
�ڸ��ݸ�˹���ɣ���Ӧ��ֻ����ʼ״̬������״̬�йأ�������أ�������������ʼ״̬������״̬��ͬ�������Ӧ����ͬ��
�ۿ��Ը�������ʽȥ��⣬��ת��xmol/LN2��
N2(g)��3H2(g) ![]() 2NH3(g)
2NH3(g)
�� 1 3 0
ת x 3x 2x
ƽ 1-x 3-3x 2x
���ݴ�ʱ������ѹǿ��Ϊԭ����7/8������ʽ��![]() ����x=0.25mol/L��
����x=0.25mol/L��
a. K= ![]() ��
��
b. N2��ƽ��ת����Ϊ0.25/1��100��=25����
c. ƽ��ʱc(N2)=0.75mol/L��c(H2)=2.25mol/L��c(NH3)=0.5mol/L��K=![]() =0.029���������ټ���2.25 molN2��0.5 mol NH3����c(N2)=3mol/L��c(H2)=2.25mol/L��c(NH3)=1mol/L��Q=
=0.029���������ټ���2.25 molN2��0.5 mol NH3����c(N2)=3mol/L��c(H2)=2.25mol/L��c(NH3)=1mol/L��Q= ![]() =0.029�����Q=K��ƽ�ⲻ�ƶ���
=0.029�����Q=K��ƽ�ⲻ�ƶ���
��2�����ݸ�˹���ɣ��跴Ӧ�����������ֱ�ΪA��B��C����B=(A-C)/3�����b=(a-c)/3������c=a-3b�����Կ�֪K3=K1/K23������4NH3(g)��6NO(g)![]() 5N2(g)��6H2O(g)��֪�÷�Ӧ�����ʾ�Ϊ���壬�������������������Ҷȱ�ʦ�S>0��
5N2(g)��6H2O(g)��֪�÷�Ӧ�����ʾ�Ϊ���壬�������������������Ҷȱ�ʦ�S>0��
��3������4NH3(g)��6NO(g)![]() 5N2(g)��6H2O(g)��֪�÷�Ӧ������������ڷ�Ӧǰ��������������¶Ȳ��䣬NH3��ת����(��)Խ��˵��ѹǿԽС�����p1<p2��a��N2��Ũ�Ȳ��ٸı䣬˵�������Ũ�ȶ����䣬���������Ϊ�ж�ƽ��ı�־����ȷ��b������6 mol N��H����ͬʱ����6 mol H��O���γɣ����ݷ�Ӧ�ص㣬���߶���ʾ����Ӧ���ʣ��������c�����ڷ�Ӧǰ������������仯����������ѹǿ���ٱ仯�����ж�ƽ�⣬��ȷ��d�����������ܶȦ�=m/V�����ڸ���ֶ������壬��Ӧǰ�����������������䣬�������㶨��������䣬����ܶ�ʼ���Ǹ���ֵ�����ʴ�ѡbd��
5N2(g)��6H2O(g)��֪�÷�Ӧ������������ڷ�Ӧǰ��������������¶Ȳ��䣬NH3��ת����(��)Խ��˵��ѹǿԽС�����p1<p2��a��N2��Ũ�Ȳ��ٸı䣬˵�������Ũ�ȶ����䣬���������Ϊ�ж�ƽ��ı�־����ȷ��b������6 mol N��H����ͬʱ����6 mol H��O���γɣ����ݷ�Ӧ�ص㣬���߶���ʾ����Ӧ���ʣ��������c�����ڷ�Ӧǰ������������仯����������ѹǿ���ٱ仯�����ж�ƽ�⣬��ȷ��d�����������ܶȦ�=m/V�����ڸ���ֶ������壬��Ӧǰ�����������������䣬�������㶨��������䣬����ܶ�ʼ���Ǹ���ֵ�����ʴ�ѡbd��


 ������״Ԫ��ҵϵ�д�
������״Ԫ��ҵϵ�д� ��ʱ�ƿ�������ϰϵ�д�
��ʱ�ƿ�������ϰϵ�д� һ��һ��һ��ͨϵ�д�
һ��һ��һ��ͨϵ�д� �㽭֮��ѧҵˮƽ����ϵ�д�
�㽭֮��ѧҵˮƽ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���о�NO2��SO2 ��CO�ȴ�����Ⱦ����Ĵ���������Ҫ���塣
��1��һ�������£���2molNO��2molO2���ں����ܱ������з������·�Ӧ��2NO(g)+O2(g)![]() 2NO2(g)�����и�����˵����Ӧ�ﵽƽ��״̬����_____________��
2NO2(g)�����и�����˵����Ӧ�ﵽƽ��״̬����_____________��
A.��ϵѹǿ���ֲ���
B.���������ɫ���ֲ���
C.NO��O2�����ʵ���֮�ȱ��ֲ���
D.ÿ����1 molO2ͬʱ����2 molNO
��2��CO�����ںϳɼ״���һ���¶��£������Ϊ2L���ܱ������м���CO��H2��������ӦCO(g)+2H2(g)![]() CH3OH(g)����ƽ����ø����Ũ�ȣ�
CH3OH(g)����ƽ����ø����Ũ�ȣ�
���� | CO | H2 | CH3OH |
Ũ�ȣ�mol/L�� | 0.9 | 1.0 | 0.6 |
�ش��������⣺
�ٻ�������ƽ����Է�������=_________________��
��ƽ�ⳣ��K=__________________��
�������������ѹ��Ϊ1L���������㣬Ԥ����ƽ����c(H2)��ȡֵ��Χ��__________��
��������������䣬�ٳ���0.6molCO��0.4molCH3OH����ʱv��______v�������������������=������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij�л����A��Ϊͬ���칹�壬���ⶨ���ǵ���Է�������С��100����1mol����O2�г��ȼ�յõ������ʵ�����CO2��H2O (g) ��ͬʱ����112L O2����״�����������������½�1mol����ȫˮ���������1mol�Һ�1mol����������һ�������£������Ա�����������Ϊ�ҡ�
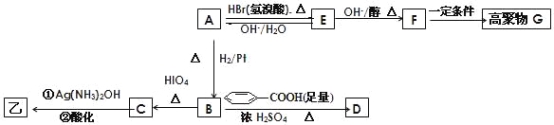
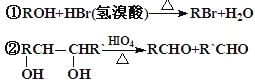
��������ײⶨ���ڼ�A�Ľṹ�ж�����C=O˫����C-O������B��HIO4���ڲ�����ʱֻ����һ�ֲ���C������Ϊ����ط�Ӧ����Ϣ��ת����ϵ��
�� ��ȷ����д���ķ���ʽ_______�������ͬ�����ʵ�ͬ���칹�干��____�֣������ף���
�� E��F �ķ�Ӧ����Ϊ_________��Ӧ��
�� A�Ľṹ��ʽΪ_________��G �Ľṹ��ʽΪ_________��
�� B��D�ķ�Ӧ��ѧ����ʽΪ_______________________________��
�� д��C���������½��з�Ӧ�Ļ�ѧ����ʽ___________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ѧ--�л���ѧ�������л���G(![]() )��һ�ֵ����㾫������������������ҵ�Ϻϳ�����·��ͼ֮һ���£�
)��һ�ֵ����㾫������������������ҵ�Ϻϳ�����·��ͼ֮һ���£�

(1)A�й����ŵ�������_____��B��C�ķ�Ӧ����Ϊ_________��
(2)G������һ����ƽ���̼ԭ����______����
(3)��ӦF��G�����ĵ�F��CH3MgBr�����ʵ���֮��Ϊ______��B��Ũ��������¼��ȿ�ͨ��һ����Ӧ�õ�E����,���ϳɲ����ô˷�������ԭ����__________��
(4)C��D�Ļ�ѧ����ʽΪ_______________��
(5)E�ж���ͬ���칹�壬������������������ͬ���칹����_____�֣�д���˴Ź���������4�����շ�����ʵĽṹ��ʽ:_______��
�ٺ���һ����Ԫ�� ��1molͬ���칹��������������Һ����������Ӧ����4 mol Ag
(6)��֪Aת��ΪB��ԭ����Fת��ΪG�����ƣ��������AΪ��ʼԭ��(�����Լ���ѡ)�Ʊ�![]() �ĺϳ�·��______________��
�ĺϳ�·��______________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��˫�ǻ���̼������ҽ���ϳ��õ�һ����������仯ѧʽ��NaAl��OH��2CO![]() �����ڸ����ʵ�˵����ȷ���ǣ� ��
�����ڸ����ʵ�˵����ȷ���ǣ� ��
A. ����������������������
B. ��������Al��OH��3��Na2CO3�Ļ����
C. 1 mol NaAl��OH��2CO3��������3 mol H![]()
D. ��ҩ�����ʺ���θ�����߷���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��A��B��C��D��Ϊ��ѧ��ѧ�г��������ʣ�����֮��ת����ϵ��ͼ(���ֲ�������ȥ)��
A![]() B
B![]() C
C
�Իش�
(1)��D�Ǿ��������Եĵ��ʣ�����������Ľ���AΪ___________(��Ԫ�ط���)
(2)��D�ǽ�����C��Һ�ڴ���ʱӦ������������D����������(�ñ�Ҫ�����ֺ����ӷ���ʽ��ʾ)______
(3)��A��B��CΪ��ͬһ�ֽ���Ԫ�ص����������������������һ���ǰ�ɫ���������Һ��A��C��Ӧ����B����д��Bת��ΪC�����п��ܵ���������ʽ_________��_________
(4)ijһ���ӷ�Ӧ��ϵ�з�Ӧ��Ͳ��ﹲ�������֣�MnO4-��H+��O2��H2O��H2O2��Mn2+��
��д����������ԭ��Ӧ�����ӷ���ʽ_______
����Һ�������Ե���ǿ�����Բ�����ǿ��Ӧѡ��_________(ѡ����ϡ����������Ũ������)����KMnO4��Һ���ữ��
���練Ӧת����0.6mo1���ӣ�������������ڱ�״�������Ϊ__________
(5)һ����Һ�п��ܺ���K+��Al3+��Mg2+��Fe2+�� H+��NH4+��Cl����HCO3����ClO����AlO2���������е������֡�Ϊ��ȷ����Һ����ɣ����������²�����
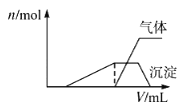
������Һ����μ���NaOH��Һ���ʵ����ȣ�������������������ʵ���(n)�����NaOH��Һ�������ϵ��ͼ��ʾ�������Һ��һ�����ڵ�������_______��һ�������ڵ�������________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����Դ������������������Դ���õ��ǵ����������Ż��⡣�������ѧ��ѧ֪ʶ�ش��������⣺
��1�������ϰ�װ��ת��������ʹ����β���е���Ҫ��Ⱦ�CO��NOx��̼�⻯����������Ӧ�����������ʣ���������β����Ⱦ��
��֪��N2(g) + O2(g)��2NO(g) ��H��+180.5 kJ �� mol��1��
2C(s)+ O2(g)��2CO(g) ��H����221.0 kJ �� mol��1��
C(s)+ O2(g)��CO2(g) ��H����393.5 kJ �� mol��1��
��β��ת����Ӧ2NO(g) +2CO(g)��N2(g)+2CO2(g)����H��________________��
��2������β�������Ƕ�CO�ĺ�����������ȼ�ϵ��Ϊ����ԭ������װ������ͼ��ʾ���õ���е����Ϊ�����ƣ������ƣ�����O2-�����ڹ�������������ƶ���

����˵������ȷ����_____________(����ĸ���)��
A�������ĵ缫��ӦʽΪ��CO + O2���D2e����CO2
B������ʱ�����ɵ缫aͨ������������缫b
C������ʱ�缫b��������O2���ɵ缫aͨ�����������缫bǨ��
D����������ͨ���ĵ���Խ��β����CO�ĺ���Խ��
��3��ij���᳧���ü״�������ˮ����һ�������£����ˮ�м���CH3OH����HNO3��ԭ��N2�����÷�Ӧ����32 g CH3OHת��6 mol���ӣ���μӷ�Ӧ�Ļ�ԭ���������������ʵ���֮��Ϊ______________��
��4��ú�ļ��Һ������ת��ΪCO��H2�����ڴ��������ºϳɼ״�������һ���¶��£���1 L�ܱ������м���CO��H2��������ӦCO(g)+2H2(g)![]() CH3OH(g)����10 min��Ӧ�ﵽƽ��ʱ��ø���ֵ�Ũ�����£�
CH3OH(g)����10 min��Ӧ�ﵽƽ��ʱ��ø���ֵ�Ũ�����£�
�� �� | CO | H2 | CH3OH |
Ũ��/(mol��L��1) | 1.2 | 1.0 | 0.6 |
�ٸ÷�Ӧ��ƽ�ⳣ������ʽΪ��K��_____________________��
�ڸ�ʱ���ڷ�Ӧ������(H2)��_________________��
��ƽ��ʱCO��ת����Ϊ_________________(����1λС��)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij��Һ�п��ܺ���K+��NH4+��Ba2+��SO42-��I-��Cl-��NO3-�еļ��֣�������Һ�ֳ����ȷݡ���������ʵ�飺��AgClʽ��Ϊ143.5��AgIʽ��Ϊ235��
����һ����Һ�м�������NaOH�����ȣ����ռ�����״̬�µ�����1.12L��
������һ����Һ�м�������Ba(NO3)2��Һ���а�ɫ�������������˵õ�����2.33g��
���ڢڵ���Һ�м�������AgNO3��Һ������4.7g����������
�йظ���Һ���������ࣨ������H+��OH-�����ж���ȷ����
A.��Һ��������2��������
B.ֻ��ȷ����Һ��NH4+��SO42-�Ƿ����
C.��Һ�������4��������
D.��Һ�в�����ͬʱ����K+��NO3-
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������������ѧ�����÷����ǿط�����״������������Ч��ʩ������˵����ȷ����
A.40%�ļ�ȩ��Һ���������;�������
B.����ҽ�ÿ��ֵ���Ҫԭ���Ǿ۱�ϩ��PP��������ʽΪ(CH3CH=CH2)n��
C.95%���Ҵ���Һ��84����Һ��ֱ����������������
D.Ϊ����ֱ�������ĭ�γɵ����ܽ���Ⱦ�����ļ��ʣ��Ͳ�ʱ���˼������ӦΪ1��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com