
���ݵ���������Ӧ���Ȼ�ѧ����ʽ��
(��)I2(g)+H2(g) 2HI(g) ��H=-9.48 kJ��mol-1
2HI(g) ��H=-9.48 kJ��mol-1
(��)I2(s)+H2(g) 2HI(g) ��H=+26.48 kJ��mol-1
2HI(g) ��H=+26.48 kJ��mol-1
�����ж���ȷ���ǣ� ��
A.254 g I2(g)��ͨ��2 g H2(g)����Ӧ����9.48 kJ
B.1 mol��̬����1 mol��̬���������������17.00 kJ
C.��Ӧ(��)�IJ���ȷ�Ӧ(��)�IJ����ȶ�
D.��Ӧ(��)�ķ�Ӧ���������ȷ�Ӧ(��)�ķ�Ӧ����������

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����A��B��C��D��E��F��G��H��I��J��K������ͼת����ϵ����������D��EΪ���ʣ��Իش�
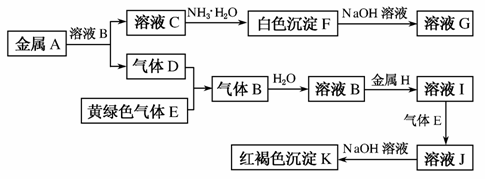
(1)д���������ʵĻ�ѧʽ��A________��B________��D________��K________��
(2)д����Ӧ��C��F�������ӷ���ʽ��____________________________________________________________________________��
(3)д����Ӧ��F��G���Ļ�ѧ����ʽ��__________________________________________________________________________________��
(4)д����Ӧ��J��K�������ӷ���ʽ��__________________________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����ʵ�������������ȷ���ǣ� ��
A. ��������ˮ�еμ�������ɫʯ����Һ���������Һ�ʺ�ɫ
B. ͭ��������ȼ��������ɫ��
C. �������費���κ��ᷴӦ������ʯӢ������������
D. ����[KAl(SO4)2��12H2O]��ˮ�����γ�Al(OH)3���壬��������ˮ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�������й�ʵ��װ�ý�����Ӧʵ�飬������ǣ� ��
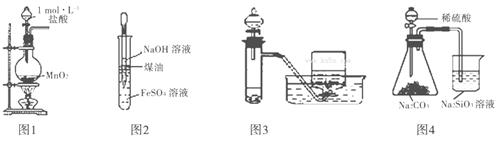
A����ͼ1��ʾװ��ʵ������ȡ����Cl2
B����ͼ2��ʾװ����ȡ���۲�Fe(OH)2
C����ͼ3��ʾװ����H2O2��Һ�Ʊ�O2
D����ͼ4��ʾװ�ñȽ�S��C��Si�ķǽ�����ǿ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
NԪ���ж��ֻ��������֮����Է���ת�����磺N2H4��HNO2��2H2O+HN3����ش��������⣺
��1��N��O�縺�Խϴ���� ��
��2��NaN3�ľ��������� ��
��3����������HN3�ڳ�������һ��Һ�壬�е�ϸߣ�Ϊ308.8K����Ҫԭ���� ��
��4��N2H4�е�Nԭ�ӵ��ӻ������� ��
��5��NO2��������һ�ֺܺõ���λ�壬���ṩ�µ��Ӷ��ǣ� ��
A����ԭ�� B����ԭ�� C�����߶�����
NO2��������ͨ����λ���γɵ�[Co(NO2)6]3������K�����ӽ�����ɻ�ɫK3[Co(NO2)6]�������˷��������ڼ�����Һ�е�K�����ӣ�д����������������ӵĵ����Ų�ʽ: ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�о�NOx��SO2��CO�ȴ�����Ⱦ����Ĵ�������������Ҫ���塣
��1��������CO��SO2�̵�����Ⱦ��һ�ַ����ǽ����ڴ���������ת��Ϊ����S����֪��
��CO(g)+ O2(g)====CO2(g) ��H=-283.0 kJ��mol-1
O2(g)====CO2(g) ��H=-283.0 kJ��mol-1
��S(s)+ O2(g)==== SO2(g) ��H=-296.0 kJ��mol-1
�˷�Ӧ���Ȼ�ѧ����ʽ��_________________________��
��2��������������ɹ⻯ѧ�����ͳ�������ĵ���Ҫ���塣
��֪����CO(g)+NO2(g)====NO(g)+CO2(g) ��H=-akJ��mol-1(a>0)
��2CO(g)+2NO(g)= ===N2(g)+
===N2(g)+ 2C
2C O2(g) ��H=-bkJ��
O2(g) ��H=-bkJ�� mol-1(b>0)
mol-1(b>0)
���ñ�״����3.36 L CO��ԭNO2��N2(CO��ȫ��Ӧ)������������ת�Ƶ��ӵ����ʵ���Ϊ________mol���ų�������Ϊ________kJ(�ú���a��b�Ĵ���ʽ��ʾ)��
��3����CH4����ԭNOxҲ�������������������Ⱦ�����磺
��CH4(g)+4NO2(g)====4NO(g)+CO2(g)+2H2O(g) ��H1=-574 kJ��mol-1��
��CH4(g)+4NO(g )====2N2(g)+CO2(g)+2H2O(g)����H2=����
)====2N2(g)+CO2(g)+2H2O(g)����H2=����
��1 mol CH4��ԭNO2��N2���������зų�������Ϊ867 kJ����H2=________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����й��������ʵ�˵���������(����)
A�����ȶ��ԣ�HCl>HI B��ԭ�Ӱ뾶��Na>Mg
C�����ԣ� H2SO3>H2SO4 D���������������S2��>Cl��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���ֶ�����Ԫ��A��B��C��D��E��ԭ��������������A��Cͬ�壬B��D ͬ�壬C���Ӻ�B���Ӿ�����ͬ�ĵ��Ӳ�ṹ��A��B��D��E�����γɹ����ͻ����A��B�γɵĻ�������ˮ�гʼ��ԣ�C��E�γɵĻ�������ˮ�г����ԡ��ش��������⣺
��1������Ԫ���У�ԭ�Ӱ뾶������ ���ǽ�������ǿ���� ����Ԫ�ط��ţ���
��2����A��B��D��E���γɵĹ����ͻ������У����ȶ��������� ���û�ѧʽ��ʾ����
��3��A��E�γɵĻ�������A��B�γɵĻ����ﷴӦ������Ļ�ѧʽΪ �����д��ڵĻ�ѧ������Ϊ ��
��4��D����������ˮ����Ļ�ѧʽΪ ��
��5������D�ڳ���ĵ���E��ȼ�գ���Ӧ�Ļ�ѧ����ʽΪ ��D�ڲ������E��ȼ�գ����ɵ���Ҫ����Ļ�ѧʽΪ ��
��6������E��ˮ��Ӧ�����ӷ���ʽΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��һ��������A��B��Ӧ������C��D���������仯��ͼ��


�����йط�ӦA��B===C��D��˵����ȷ���� (����)��
A����Ӧǰ��ԭ�ӵ��������Ŀһ������
B����Ӧǰ����ӵ��������Ŀһ���ı�
C����Ӧ���������E1���������������E2һ�����
D���˷�Ӧһ���������ı仯
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com