
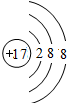
AΓΔBΓΔCΓΔDΥΡ÷÷‘ΣΥΊΒΡ‘≠Ή”–ρ ΐΕΦ–Γ”Ύ20Θ§A”κBΆ§÷ςΉεΘ§B”κCΆ§÷ήΤΎΘ§0.2 molΒΡCΒΞ÷ ”κΉψΝΩœΓH2SO4Ζ¥”Π ±Ζ≈≥ωH2ΓΓ6.72 L(±ξΉΦΉ¥Ωω)Θ§A‘≠Ή”ΉνΆβ≤ψ…œΒΡΒγΉ” ΐ±»¥ΈΆβ≤ψ…œΒΡΒγΉ” ΐΕύ5ΗωΘ§C”κDΒΡ‘≠Ή”–ρ ΐ÷°ΚΆΈΣ32Θ°
(1)AΓΔBΓΔCΓΔDΒΡ‘ΣΥΊΖϊΚ≈Ζ÷±πΈΣΘΚ________ΓΔ________ΓΔ________ΓΔ________Θ°
(2)AΒΡάκΉ”ΫαΙΙ Ψ“βΆΦΈΣ________Θ§CΒΡ‘≠Ή”ΫαΙΙ Ψ“βΆΦΈΣ________Θ°
(3)B‘≠Ή”ΒΡΒγΉ” ΫΈΣ________Θ§A”κDΒΡΜ·ΚœΈοΒΡΒγΉ” ΫΈΣ________Θ°
(4)CΒΡΗΏΦέ―θΜ·ΈοΕ‘”ΠΥ°Μ·ΈοΒΡΜ·―ß ΫΈΣ________ΘΜΥϋΗζ―ΈΥαΖ¥”ΠΒΡάκΉ”ΖΫ≥Χ Ϋ «________ΘΜΗζ…’Φν»ή“ΚΖ¥”ΠΒΡάκΉ”ΖΫ≥Χ Ϋ «________________Θ°
 ΧλΧλœρ…œ“Μ±ΨΚΟΨμœΒΝ–¥πΑΗ
ΧλΧλœρ…œ“Μ±ΨΚΟΨμœΒΝ–¥πΑΗ –Γ―ß…ζ10Ζ÷÷””Π”ΟΧβœΒΝ–¥πΑΗ
–Γ―ß…ζ10Ζ÷÷””Π”ΟΧβœΒΝ–¥πΑΗ
| ΡξΦΕ | ΗΏ÷–ΩΈ≥Χ | ΡξΦΕ | ≥θ÷–ΩΈ≥Χ |
| ΗΏ“Μ | ΗΏ“ΜΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ | ≥θ“Μ | ≥θ“ΜΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ |
| ΗΏΕΰ | ΗΏΕΰΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ | ≥θΕΰ | ≥θΕΰΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ |
| ΗΏ»ΐ | ΗΏ»ΐΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ | ≥θ»ΐ | ≥θ»ΐΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ |
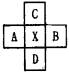
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ

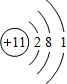
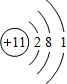
| ||
| ||
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚΈοάμΫΧ―– “ Χβ–ΆΘΚ013
A. 4x
B. 4xΘΪ6
C. 4xΘΪ10
D. 4xΘΪ14
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ013
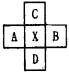
‘≠Ή”–ρ ΐΈΣJΒΡ‘ΣΥΊ…υ÷ήΤΎ±μ÷–ΈΜ”ΎAΓΔBΓΔCΓΔDΥΡ÷÷‘ΣΥΊΒΡ÷–ΦδΘ§AΓΔBΓΔCΓΔDΥΡ÷÷‘ΣΒΡ «Θ®ογœΒΓΔοΙœΒΘ§ΝψΉε‘ΣΥΊ≥ΐΆβΘ©Γ≠Θ® Θ©
A. 4x
B. 4xΘΪ6
C. 4xΘΪ10
D. 4xΘΪ14
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ
AΓΔBΓΔCΓΔDΥΡ÷÷ΕΧ÷ήΤΎ‘ΣΥΊΘ§‘≠Ή”–ρ ΐD>A>B>CΘ§«“AΓΔBΆ§÷ήΤΎΘ§CΓΔDΆ§÷ςΉεΘ§AΒΡ‘≠Ή”ΫαΙΙ Ψ“βΆΦΈΣΘΚ 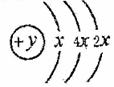 Θ§BΓΔCΩ…–Έ≥…άκΉ”Μ·ΚœΈο
Θ§BΓΔCΩ…–Έ≥…άκΉ”Μ·ΚœΈο![]() Θ§Ψί¥ΥΧνΩ’ΘΚ
Θ§Ψί¥ΥΧνΩ’ΘΚ
![]() (1)AΒΡ‘ΣΥΊΟϊ≥ΤΈΣ Θ§ΤδΤχΧ§―θΜ·ΈοΒΡΜ·―ß ΫΈΣ ΓΘ
(1)AΒΡ‘ΣΥΊΟϊ≥ΤΈΣ Θ§ΤδΤχΧ§―θΜ·ΈοΒΡΜ·―ß ΫΈΣ ΓΘ
![]() (2)AΓΔBΓΔCΓΔDΥΡ÷÷‘ΣΥςΒΡ‘≠”ηΘ§ΑκΨΕ”…–ΓΒΫ¥σΒΡΥ≥–ρΈΣ ΓΘ
(2)AΓΔBΓΔCΓΔDΥΡ÷÷‘ΣΥςΒΡ‘≠”ηΘ§ΑκΨΕ”…–ΓΒΫ¥σΒΡΥ≥–ρΈΣ ΓΘ
![]() (3)BΚΆCΉνΗΏΦέ―θΜ·ΈοΒΡΥ°Μ·ΈοΜ·―ß ΫΖ÷±πΈΣ ΚΆ ΓΘ
(3)BΚΆCΉνΗΏΦέ―θΜ·ΈοΒΡΥ°Μ·ΈοΜ·―ß ΫΖ÷±πΈΣ ΚΆ ΓΘ
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ
(6Ζ÷)AΓΔBΓΔCΓΔDΥΡ÷÷ΕΧ÷ήΤΎ‘ΣΥΊΘ§‘≠Ή”–ρ ΐD>A>B>CΘ§«“AΓΔBΆ§÷ήΤΎΘ§CΓΔDΆ§÷ςΉεΘ§AΒΡ‘≠Ή”ΫαΙΙ Ψ“βΆΦΈΣΘΚ 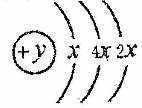 Θ§BΓΔCΩ…–Έ≥…άκΉ”Μ·ΚœΈο
Θ§BΓΔCΩ…–Έ≥…άκΉ”Μ·ΚœΈο![]() Θ§Ψί¥ΥΧνΩ’ΘΚ
Θ§Ψί¥ΥΧνΩ’ΘΚ
(1)AΒΡ‘ΣΥΊΟϊ≥ΤΈΣ Θ§ΤδΤχΧ§―θΜ·ΈοΒΡΜ·―ß ΫΈΣ ΓΘ
(2)AΓΔBΓΔCΓΔDΥΡ÷÷‘ΣΥςΒΡ‘≠”ηΘ§ΑκΨΕ”…–ΓΒΫ¥σΒΡΥ≥–ρΈΣ ΓΘ
(3)BΚΆCΉνΗΏΦέ―θΜ·ΈοΒΡΥ°Μ·ΈοΜ·―ß ΫΖ÷±πΈΣ ΚΆ ΓΘ
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΑΌΕ»÷¬–≈ - ΝΖœΑ≤αΝ–±μ - ‘ΧβΝ–±μ
Κΰ±± ΓΜΞΝΣΆχΈΞΖ®ΚΆ≤ΜΝΦ–≈œΔΨΌ±®ΤΫΧ® | Άχ…œ”–ΚΠ–≈œΔΨΌ±®Ή®«χ | Βγ–≈’©Τ≠ΨΌ±®Ή®«χ | …φάζ Ζ–ιΈό÷ς“ε”–ΚΠ–≈œΔΨΌ±®Ή®«χ | …φΤσ«÷»®ΨΌ±®Ή®«χ
ΈΞΖ®ΚΆ≤ΜΝΦ–≈œΔΨΌ±®ΒγΜΑΘΚ027-86699610 ΨΌ±®” œδΘΚ58377363@163.com