
ЎҫМвДҝЎҝПВБРЛө·ЁХэИ·өДКЗ(ЎЎЎЎ)
ўЩұкЧјЧҙҝцПВЈ¬6.02ЎБ1023ёц·ЦЧУЛщХјөДМе»эФјКЗ22.4 L
ўЪ0.5 mol H2ЛщХјМе»эОӘ11.2 L
ўЫұкЧјЧҙҝцПВЈ¬1 mol H2OөДМе»эОӘ22.4 L
ўЬұкЧјЧҙҝцПВЈ¬28 g COУлN2өД»мәПЖшМеөДМе»эФјОӘ22.4 L
ўЭёчЦЦЖшМеөДЖшМеДҰ¶ыМе»э¶јФјОӘ22.4 LЎӨmolЈӯ1
ўЮұкЧјЧҙҝцПВЈ¬Ме»эПаН¬өДЖшМеөД·ЦЧУКэПаН¬
A. ўЩўЫўЭB. ўЬўЮC. ўЪўЬўЮD. ўЩўЬўЮ
Ўҫҙр°ёЎҝB
ЎҫҪвОцЎҝ
ўЩёГОпЦКІ»Т»¶ЁОӘЖшМеЈ¬ИфОӘЖшМеКұЈ¬ұкЧјЧҙҝцПВЈ¬6.02ЎБ1023ёц·ЦЧУЛщХјөДМе»эФјКЗ![]() molЎБ22.4L/mol=22.4LЈ¬№КҙнОуЈ»
molЎБ22.4L/mol=22.4LЈ¬№КҙнОуЈ»
ўЪІ»Т»¶ЁФЪұкЧјЧҙҝцПВЈ¬ФтVmОҙЦӘЈ¬І»ДЬАыУГV=nVmјЖЛгЈ¬№КҙнОуЈ»
ўЫұкЧјЧҙҝцПВЈ¬Л®І»КЗЖшМеЈ¬І»ДЬёщҫЭV=nVmјЖЛгЈ¬№КҙнОуЈ»
ўЬCOУлN2өДДҰ¶ыЦКБҝҫщОӘ28g/molЈ¬ұкЧјЧҙҝцПВЈ¬28g COУлN2өД»мәПЖшМеөДМе»эФјОӘ![]() ЎБ22.4L/mol=22.4LЈ¬№КХэИ·Ј»
ЎБ22.4L/mol=22.4LЈ¬№КХэИ·Ј»
ўЭЖшМеДҰ¶ыМе»эУлОВ¶ИЎўС№ЗҝУР№ШЈ¬ЧҙМ¬ОҙЦӘЈ¬І»ДЬИ·¶ЁЖшМеөДДҰ¶ыМе»эЈ¬ұкЧјЧҙҝцПВЈ¬ёчЦЦЖшМеөДЖшМеДҰ¶ыМе»э¶јФјОӘ22.4Lmol-1Ј¬№КҙнОуЈ»
ўЮұкЧјЧҙҝцПВЈ¬Ме»эПаН¬өДЖшМеЈ¬ОпЦКөДБҝПаН¬Ј¬УЙn=![]() ҝЙЦӘЈ¬·ЦЧУКэПаН¬Ј¬№КХэИ·Ј»
ҝЙЦӘЈ¬·ЦЧУКэПаН¬Ј¬№КХэИ·Ј»
№КСЎBЎЈ


 ГыРЈҝОМГПөБРҙр°ё
ГыРЈҝОМГПөБРҙр°ё
| Дкј¶ | ёЯЦРҝОіМ | Дкј¶ | іхЦРҝОіМ |
| ёЯТ» | ёЯТ»Гв·СҝОіМНЖјцЈЎ | іхТ» | іхТ»Гв·СҝОіМНЖјцЈЎ |
| ёЯ¶ю | ёЯ¶юГв·СҝОіМНЖјцЈЎ | іх¶ю | іх¶юГв·СҝОіМНЖјцЈЎ |
| ёЯИэ | ёЯИэГв·СҝОіМНЖјцЈЎ | іхИэ | іхИэГв·СҝОіМНЖјцЈЎ |
ҝЖДҝЈәёЯЦР»ҜС§ АҙФҙЈә МвРНЈә
ЎҫМвДҝЎҝПВБРРрКцХэИ·өДКЗ
ўЩСх»Ҝ»№Фӯ·ҙУҰөДКөЦККЗөзЧУөДөГК§ЎЈ
ўЪИф1 molЖшМеөДМе»эОӘ22.4 LЈ¬ФтЛьТ»¶ЁҙҰУЪұкЧјЧҙҝцПВЎЈ
ўЫұкЧјЧҙҝцПВЈ¬1 L HClәН1 L H2OөДОпЦКөДБҝПаН¬ЎЈ
ўЬИЬУЪЛ®өГөҪөДИЬТәҝЙТФөјөзөДОпЦКҫНКЗөзҪвЦКЎЈ
ўЭАыУГ¶Ўҙп¶ыПЦПуЗшұрИЬТәәНҪәМеЎЈ
ўЮБҪЦЦОпЦКөДОпЦКөДБҝПаН¬Ј¬ФтЛьГЗФЪұкЧјЧҙҝцПВөДМе»эТІПаН¬ЎЈ
ўЯФЪН¬ОВН¬Ме»эКұЈ¬ЖшМеОпЦКөДОпЦКөДБҝФҪҙуЈ¬ФтС№ЗҝФҪҙуЎЈ
ўаН¬ОВН¬С№ПВЈ¬ЖшМеөДГЬ¶ИУлЖшМеөДПа¶Ф·ЦЧУЦКБҝіЙХэұИЎЈ
A.ўЩўЪўЫўЬB.ўЪўЫўЮўЯўаC.ўЭўЯўаD.ўЬўЭўЯўа
Ійҝҙҙр°ёәНҪвОц>>
ҝЖДҝЈәёЯЦР»ҜС§ АҙФҙЈә МвРНЈә
ЎҫМвДҝЎҝПВБРёчЧйКэҫЭЦРЈ¬З°ХЯёХәГКЗәуХЯБҪұ¶өДКЗ (ЎЎЎЎ)
A. 2 mol H2OөДДҰ¶ыЦКБҝәН1 mol H2OөДДҰ¶ыЦКБҝ
B. 200 mL 1 molЎӨL-1ВИ»ҜёЖИЬТәЦРc(Cl-)әН100 mL 2 molЎӨL-1ВИ»ҜјШИЬТәЦРc(Cl-)
C. 64 g¶юСх»ҜБтЦРСхФӯЧУКэәНұкЧјЧҙҝцПВ22.4 LТ»Сх»ҜМјЦРСхФӯЧУКэ
D. 20% NaOHИЬТәЦРNaOHөДОпЦКөДБҝЕЁ¶ИәН10% NaOHИЬТәЦРNaOHөДОпЦКөДБҝЕЁ¶И
Ійҝҙҙр°ёәНҪвОц>>
ҝЖДҝЈәёЯЦР»ҜС§ АҙФҙЈә МвРНЈә
ЎҫМвДҝЎҝХЖОХТЗЖчөДГыіЖЎўЧйЧ°ј°К№УГ·Ҫ·ЁКЗЦРС§»ҜС§КөСйөД»щҙЎЈ¬ПВНјОӘБҪМЧКөСйЧ°ЦГЎЈ
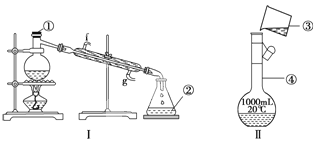
ЈЁ1Ј©РҙіцПВБРТЗЖчөДГыіЖЈәўЩ__________Ј¬ўЪ__________Ј¬
ЈЁ2Ј©ИфАыУГЧ°ЦГўс·ЦАлЛДВИ»ҜМјәНҫЖҫ«өД»мәПОпЈ¬»№ИұЙЩөДТЗЖчУР______________Ј¬Ҫ«ТЗЖчІ№ідНкХыәуҪшРРөДКөСйІЩЧчөДГыіЖОӘ____________Ј»
ЈЁ3Ј©УГNa2CO3ЎӨ10H2Oҫ§МеЈ¬ЕдЦЖ0.2 molЎӨLЈӯ1өДNa2CO3ИЬТә480 mLЎЈ
ўЩУҰіЖИЎNa2CO3ЎӨ10H2Oҫ§МеөДЦКБҝЈә__________ЎЈ
ўЪЛщУГТЗЖчіэНРЕММмЖҪЎўЙХұӯЎўІЈБ§°фЎўТ©іЧЎўБҝНІНвЈ¬»№РиТӘ__________(МоТЗЖчГыіЖ)ЎЈ
ўЫёщҫЭПВБРІЩЧч¶ФЛщЕдИЬТәөДЕЁ¶ИІъЙъөДУ°ПмЈ¬НкіЙПВБРТӘЗуЈә
AЈ®Na2CO3ЎӨ10H2Oҫ§МеК§ИҘБЛІҝ·ЦҪбҫ§Л® |
BЈ®УГЎ°ЧуВлУТОпЎұөДіЖБҝ·Ҫ·ЁіЖБҝҫ§Ме(К№УГУОВл) |
CЈ®іЖБҝМјЛбДЖҫ§МеКұЛщУГнАВлЙъРв |
DЈ®ИЭБҝЖҝОҙҫӯёЙФпК№УГ |
ЖдЦРТэЖрЛщЕдИЬТәЕЁ¶ИЖ«ёЯөДУР___________(МоЧЦДё)
ўЬЧ°ЦГўтКЗДіН¬С§ЧӘТЖИЬТәөДКҫТвНјЈ¬НјЦРөДҙнОуКЗ__________________
Ійҝҙҙр°ёәНҪвОц>>
ҝЖДҝЈәёЯЦР»ҜС§ АҙФҙЈә МвРНЈә
ЎҫМвДҝЎҝЙиNAұнКҫ°ў·ьјУөВВЮіЈКэөДЦөЈ¬ПВБРРрКцЦРХэИ·өДКЗЈЁ Ј©
A. іЈОВіЈС№ПВЈ¬11.2LСхЖшЛщә¬өДФӯЧУКэОӘNA
B. 1.8gөДNH4+АлЧУЦРә¬УРөДЦКЧУКэОӘNA
C. іЈОВіЈС№ПВЈ¬46gNO2ә¬УРөДФӯЧУЧЬКэОӘ3NA
D. ұкҝцПВЈ¬4.48LH2OЦРә¬УРЗвФӯЧУКэОӘ0.4NA
Ійҝҙҙр°ёәНҪвОц>>
ҝЖДҝЈәёЯЦР»ҜС§ АҙФҙЈә МвРНЈә
ЎҫМвДҝЎҝНӯКЗЦШТӘҪрКфЈ¬CuөД»ҜәПОпФЪҝЖС§СРҫҝәН№ӨТөЙъІъЦРҫЯУРРн¶аУГНҫЈ¬ИзCuSO4ИЬТәіЈУГЧчөзҪвТәЎўөз¶ЖТәөИЎЈЗл»ШҙрТФПВОКМвЈә
ЈЁ1Ј©ФӘЛШҪр(Au)ҙҰУЪЦЬЖЪұнЦРөДөЪ6ЦЬЖЪЈ¬УлCuН¬ЧеЈ¬ҪрФӯЧУЧоНвІгөзЧУЕЕІјКҪОӘ_________Ј»Т»ЦЦНӯәПҪрҫ§МеҫЯУРБў·ҪЧоГЬ¶С»эөДҪб№№Ј¬ФЪҫ§°ыЦРНӯФӯЧУҙҰУЪГжРДЈ¬ҪрФӯЧУҙҰУЪ¶ҘөгО»ЦГЈ¬ФтёГәПҪрЦРНӯФӯЧУУлҪрФӯЧУКэБҝЦ®ұИОӘ_____Ј¬ёГҫ§МеЦРЈ¬ФӯЧУЦ®јдөДЧчУГБҰКЗ________Ј»
ЈЁ2Ј©ЙПКцҫ§МеҫЯУРҙўЗв№ҰДЬЈ¬ЗвФӯЧУҝЙҪшИлөҪУЙНӯФӯЧУУлҪрФӯЧУ№№іЙөДЛДГжМеҝХП¶ЦРЎЈИфҪ«НӯФӯЧУУлҪрФӯЧУөИН¬ҝҙҙэЈ¬ёГҫ§МеҙўЗвәуөДҫ§°ыҪб№№УлCaF2өДҪб№№ПаЛЖЈ¬ёГҫ§МеҙўЗвәуөД»ҜС§КҪУҰОӘ_____________ЎЈ
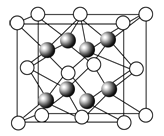
ЈЁ3Ј©CuSO4ҫ§МеөД№№іЙОўБЈКЗ____әН____Ј¬ОўБЈјдөДЧчУГБҰКЗ_______Ј¬ёГҫ§МеКфУЪ____ҫ§МеЈ»
ЈЁ4Ј©SO42ЈӯЦРSТФsp3ФУ»ҜЈ¬SO42ЈӯөДБўМе№№РНКЗ__________________________Ј»
ЈЁ5Ј©CuSO4·ЫД©іЈУГАҙјмСйТ»Р©УР»ъОпЦРөДОўБҝЛ®·ЦЈ¬ЖдФӯТтКЗ____________ЎЈ
Ійҝҙҙр°ёәНҪвОц>>
ҝЖДҝЈәёЯЦР»ҜС§ АҙФҙЈә МвРНЈә
ЎҫМвДҝЎҝСЗПхЛбДЖ№г·әУГУЪГҪИҫјБЎўЖҜ°ЧјБөИЎЈДіРЛИӨРЎЧйУГПВБРЧ°ЦГЦЖИЎҪПҙҝҫ»өДNaNO2ЎЈ
јЧ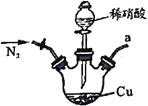 ТТ
ТТ ұы
ұы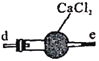 ¶Ў
¶Ў![]()
·ҙУҰФӯАнОӘЈә2NO+Na2O2=2NaNO2ЎЈТСЦӘЈәNOДЬұ»ЛбРФKMnO4Сх»ҜіЙNO3-Ј¬MnO4-ұ»»№ФӯОӘMn2+ЎЈПВБР·ЦОцҙнОуөДКЗ
A.јЧЦРөОИлПЎПхЛбЗ°РиНЁИЛN2
B.ТЗЖчөДБ¬ҪУЛіРтОӘa-f-g-d-e-b
C.ұыЦРCaCl2УГУЪёЙФпNO
D.ТТЦРОьКХОІЖшКұ·ўЙъөДАлЧУ·ҙУҰОӘ3MnO4-+5NO+4H+=3Mn2++5NO3-+2H2O
Ійҝҙҙр°ёәНҪвОц>>
ҝЖДҝЈәёЯЦР»ҜС§ АҙФҙЈә МвРНЈә
ЎҫМвДҝЎҝТСЦӘөЁ·ҜИЬУЪЛ®КұИЬТәОВ¶ИҪөөНЈ¬өЁ·Ҝ·ЦҪвөДИИ»ҜС§·ҪіМКҪОӘЈәCuSO45H2O(s)ЈҪCuSO4(s)Ј«5H2O(l) ҰӨHЈҪ+Q1kJЎӨmol-1Ј»КТОВПВЈ¬ИфҪ«1 molОЮЛ®БтЛбНӯИЬҪвОӘИЬТәКұ·ЕИИQ2kJЈ¬ФтЈЁЈ©
A. Q1>Q2 B. Q1ЈҪQ2 C. Q1<Q2 D. ОЮ·ЁұИҪП
Ійҝҙҙр°ёәНҪвОц>>
ҝЖДҝЈәёЯЦР»ҜС§ АҙФҙЈә МвРНЈә
ЎҫМвДҝЎҝДіСРҫҝРФС§П°РЎЧйУыУГ»ҜС§·Ҫ·ЁІвБҝТ»ёцІ»№жФтИЭЖчөДМе»эЈ¬°С40 g NaOH·ЕИлЙХұӯЦРЈ¬јУИлТ»¶ЁБҝөДХфБуЛ®ЎЈҙэNaOHНкИ«ИЬҪвәуЈ¬Ҫ«ИЬТәИ«ІҝЧӘТЖөҪИЭЖчЦРЈ¬УГХфБуЛ®ПЎКНЦБНкИ«ідВъИЭЖчЈ¬ҙУЦРИЎіцИЬТә100 mLЈ¬ёГИЬТәЗЎәГУл20mL 1mol/L CuSO4ИЬТәНкИ«·ҙУҰЎЈ
(1)РҙіцCuSO4өДДҰ¶ыЦКБҝ______________ЎЈ
(2)ЗуЕдЦЖөДNaOHИЬТәөДОпЦКөДБҝЕЁ¶И______________ЎЈ
(3)КФјЖЛгёГИЭЖчөДМе»э______________ЎЈ
Ійҝҙҙр°ёәНҪвОц>>
°Щ¶ИЦВРЕ - Б·П°ІбБРұн - КФМвБРұн
әюұұКЎ»ҘБӘНшОҘ·ЁәНІ»БјРЕПўҫЩұЁЖҪМЁ | НшЙПУРәҰРЕПўҫЩұЁЧЁЗш | өзРЕХ©ЖӯҫЩұЁЧЁЗш | ЙжАъК·РйОЮЦчТеУРәҰРЕПўҫЩұЁЧЁЗш | ЙжЖуЗЦИЁҫЩұЁЧЁЗш
ОҘ·ЁәНІ»БјРЕПўҫЩұЁөз»°Јә027-86699610 ҫЩұЁУКПдЈә58377363@163.com