

| O | 3 |
| O | 3 |
| O | 2 |
| 4.8g |
| 160g/mol |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��15�֣���ˮ����ͭ�ڼ����������ܷ����ֽⷴӦ����������ͭ�������������������������ijѧ����ͼ����ͼ��ʾװ����ȷ���û�ѧ��Ӧ�и����ʵļ�����ϵ
�Իش�
��1�����ȹ����У��Թ�A�з�����ʵ������Ϊ ��
��2��װ��E��F�������� ��
��3����ѧ��ʹ��װ��B�ı����dz�ȥ��������е������������ᴿ����������������ȷ
��Ϊʲô��
��4����ѧ����������װ�ý�һ����������ˮ����ͭ����A�м���ʹ��ֽ⣬�������
�������ƫС����ԭ������������� ��������ţ�
A����ˮ����ͭδ��ȫ�ֽ�o*m
B��ʵ�����ʱװ��A�в���������
C�� ��
��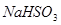 ��Һ����ʱ������
��Һ����ʱ������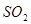 ����
����
D��������Ͳ�еĶ���ʱ��E�е�Һ�����F�е�Һ��
��5����һѧ����4.8g��ˮ����ͭ��ּ���ʹ����ȫ�ֽ������ȷ��ʵ�鷽����ȥ���������еĶ������������������������������Ϊ224mL����״�������ݴ˿ɼ������������Ϊ mol����������Ϊ mol��
��6��������ʵ�����ݿ�֪��ˮ����ͭ���ȷֽ�Ļ�ѧ����ʽΪ��
___________________________________________________________________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010�꽭��ʡ�ϸ߶��и����������������ۺϣ���ѧ���� ���ͣ�ʵ����
��15�֣���ˮ����ͭ�ڼ����������ܷ����ֽⷴӦ����������ͭ�������������������������ijѧ����ͼ����ͼ��ʾװ����ȷ���û�ѧ��Ӧ�и����ʵļ�����ϵ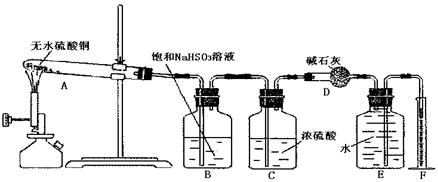
�Իش�
��1�����ȹ����У��Թ�A�з�����ʵ������Ϊ ��
��2��װ��E��F�������� ��
��3����ѧ��ʹ��װ��B�ı����dz�ȥ��������е������������ᴿ����������������ȷ
��Ϊʲô��
��4����ѧ����������װ�ý�һ����������ˮ����ͭ����A�м���ʹ��ֽ⣬�������
�������ƫС����ԭ������������� ��������ţ�
| A����ˮ����ͭδ��ȫ�ֽ�o*m |
| B��ʵ�����ʱװ��A�в��������� |
C��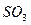 �� ��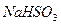 ��Һ����ʱ������ ��Һ����ʱ������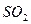 ���� ���� |
| D��������Ͳ�еĶ���ʱ��E�е�Һ�����F�е�Һ�� |
 �����������Ϊ224mL����״�������ݴ˿ɼ������������Ϊ mol����������Ϊ mol��
�����������Ϊ224mL����״�������ݴ˿ɼ������������Ϊ mol����������Ϊ mol���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010�꽭��ʡ�����������������ۺϣ���ѧ���� ���ͣ�ʵ����
��15�֣���ˮ����ͭ�ڼ����������ܷ����ֽⷴӦ����������ͭ�������������������������ijѧ����ͼ����ͼ��ʾװ����ȷ���û�ѧ��Ӧ�и����ʵļ�����ϵ

�Իش�
��1�����ȹ����У��Թ�A�з�����ʵ������Ϊ ��
��2��װ��E��F�������� ��
��3����ѧ��ʹ��װ��B�ı����dz�ȥ��������е������������ᴿ����������������ȷ
��Ϊʲô��
��4����ѧ����������װ�ý�һ����������ˮ����ͭ����A�м���ʹ��ֽ⣬�������
�������ƫС����ԭ������������� ��������ţ�
A����ˮ����ͭδ��ȫ�ֽ�o*m
B��ʵ�����ʱװ��A�в���������
C�� ��
�� ��Һ����ʱ������
��Һ����ʱ������ ����
����
D��������Ͳ�еĶ���ʱ��E�е�Һ�����F�е�Һ��
��5����һѧ����4.8g��ˮ����ͭ��ּ���ʹ����ȫ�ֽ������ȷ��ʵ�鷽����ȥ���������еĶ������������������������������Ϊ224mL����״�������ݴ˿ɼ������������Ϊ mol����������Ϊ mol��
��6��������ʵ�����ݿ�֪��ˮ����ͭ���ȷֽ�Ļ�ѧ����ʽΪ��
___________________________________________________________________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010�꽭��ʡ�˴����ϸ߶��и߿���ѧģ���Ծ��������棩 ���ͣ������

 ��
��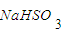 ��Һ����ʱ������
��Һ����ʱ������ ����
�����鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com